LESSON 91: Mole to Mole
THINK ABOUT IT
462
Water treatment plants in our communities process millions of gallons of water a day. These plants remove toxic substances from the water through a series of chemical and physical treatments. For example, impure water often contains dissolved metal cations. These can be removed from water through precipitation of insoluble metal salts. Once the metals are in solid form, they can be safely separated by filtration. Of course, for maximum effectiveness, the treatment plant should remove as much of each metal from the water as possible.

How can you convert all the reactants to products?
To answer this question, you will explore
Moles of Product
Excess and Limiting Reactant
Moles of Product
EXPLORING THE TOPIC
Moles of Product
The list of metals that can show up in our water supplies is quite long, and it includes lead, silver, mercury, chromium, copper, zinc, cadmium, and tin. According to the solubility table in the previous lesson, many metals form hydroxides and carbonates that are not soluble. So a good way to remove the more toxic metal cations from our water supplies is to add hydroxides or alkali metal carbonates to precipitate hazardous metal cations.
Imagine a water sample that contains unwanted copper ions. The goal is to figure out how to remove as many of those ions per liter of solution as possible through precipitation. Sodium hydroxide, NaOH, is chosen as a reactant because it is soluble and contains a hydroxide ion. When hydroxide ions enter the solution, solid copper (II) hydroxide, Cu(OH)2, will precipitate. The question remains: How much NaOH should be added, and in what concentration?
463
The next illustration shows what happens when a solution of 1.0 M CuSO4 is mixed with a solution of 1.0 M NaOH in different ratios. Take a moment to examine the outcomes.
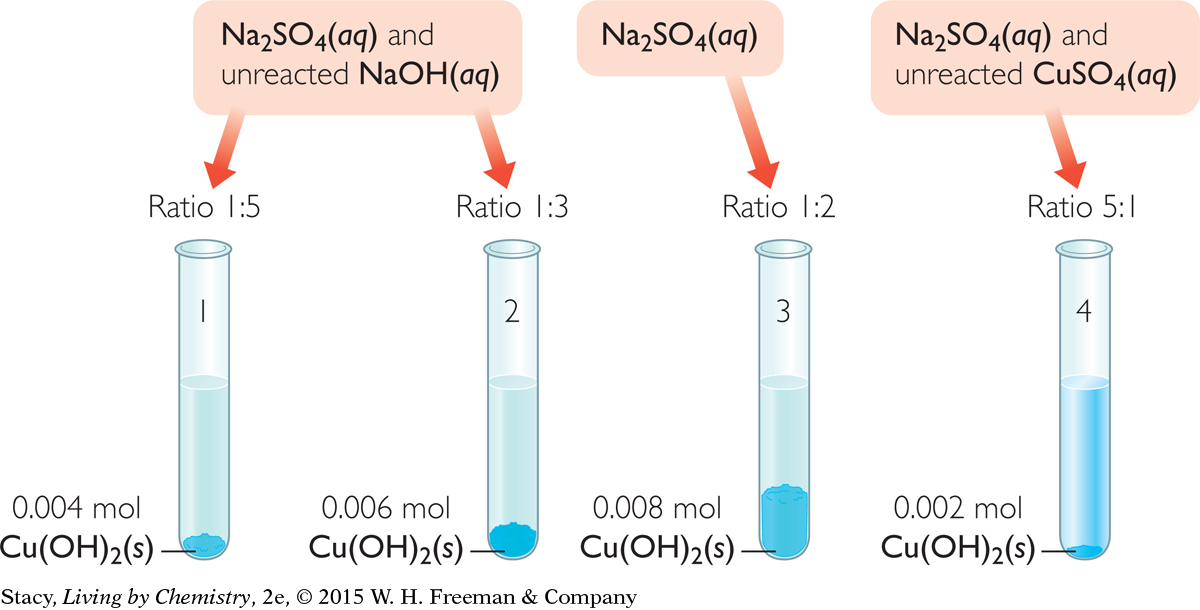
In each case, a total of 24 mL of solution are combined. However, the amounts of 1.0 M CuSO4 and 1.0 M NaOH that are combined vary from test tube to test tube. The combination that produces the maximum amount of solid precipitate is in test tube 3, where 8 mL of 1.0 M CuSO4 combined with 16 mL of 1.0 M NaOH. This results in 0.008 mol of solid copper hydroxide.
After the reaction, all four test tubes contain aqueous sodium sulfate, Na2SO4(aq), and a blue solid, copper (II) hydroxide, Cu(OH)2(s). Therefore, sodium sulfate and copper (II) hydroxide must be products of this reaction. The solutions in three of the test tubes are almost colorless, while the solution in test tube 4 is pale blue. This means there is unreacted CuSO4(aq) left over in the last test tube.
Chemical equation: CuSO4(aq) + 2NaOH(aq) → Cu(OH)2(s) + Na2SO4(aq)
MOLE RATIO
The coefficients in a chemical equation indicate the proportions in which substances react, in moles or other counting units. The coefficients in this particular equation indicate that for every 1 mol of CuSO4, 2 mol of NaOH are required to make the maximum amount of products. The test tube containing the most amount of solid (test tube 3) corresponds to this 1:2 ratio of reactants. This ratio is often referred to as the mole ratio. So, using the ratio given by the balanced chemical equation produces the maximum amount of product.
Big Idea
Big Idea
To get the maximum amount of product from a reaction, reactants must be mixed in the correct proportions.
464
Example 1
Precipitation of Silver Hydroxide
An aqueous solution contains 0.020 mol of silver nitrate, AgNO3(aq). Predict the number of moles of NaOH(aq) you will need to precipitate all of the silver, Ag, as silver hydroxide, AgOH.
Solution
First, write a balanced chemical equation for the reaction.
AgNO3(aq) + NaOH(aq) → AgOH(s) + NaNO3(aq)
The mole ratio of AgNO3 to NaOH is 1:1.
The ratio given by the balanced equation is 1:1, and you have 0.020 mol of AgNO3. So you will need 0.020 mol of NaOH to precipitate all of the silver.
Excess and Limiting Reactant
Excess and Limiting Reactant
Look again at the four test tubes. Using the molarity and volume data for each reactant, you can determine the number of moles of each reactant. The table shows the moles of each reactant and the ratios in which they are combined.
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
One of the best ways to test for the presence of heavy metals in a person’s body is through hair samples. Human hair is a permanent record of some of the substances that have passed through someone’s body. A three-inch strand of hair will give a six-month history of what’s going on chemically in the body.
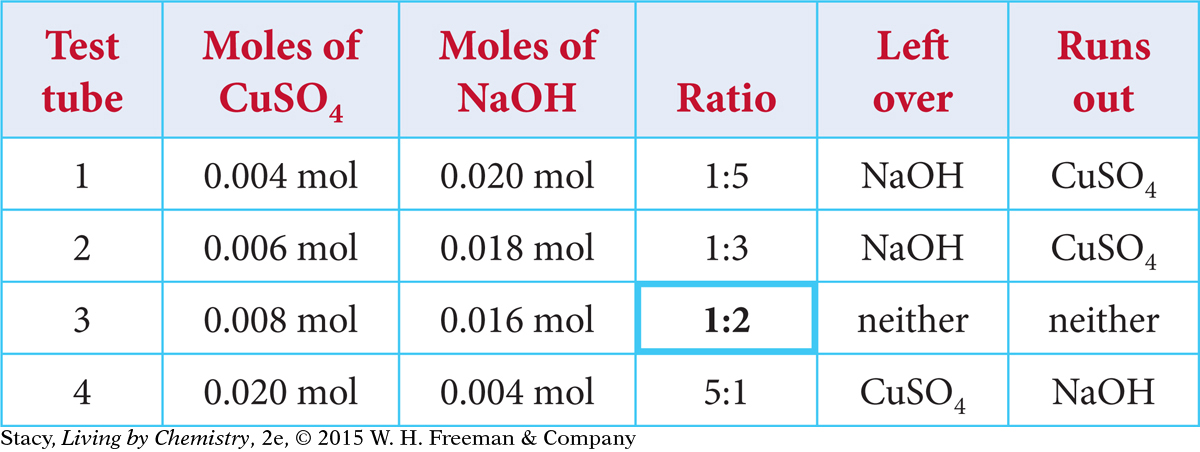
Notice that in test tubes 1, 2, and 4, one of the reactants runs out before the other. Only in test tube 3 have both reactants been used up completely. Test tubes 1 and 2 no longer have copper ions in solution, but the solution is now toxic due to the excess NaOH. Test tube 4 still has copper ions in solution.
If you mix the reactants in a ratio other than the mole ratio, a reaction will still occur, but one of the reactants will run out and the other one will have some left over. The reactant that runs out is called the limiting reactant, or limiting reagent. This is because it limits how much product you can make.
To remove all of the copper ions from the solution, it is best to mix the reactants in the mole ratio determined by the balanced chemical equation.
If you want to remove copper ions (or any other metal ions) from water in a water treatment plant, it would be best to add sodium hydroxide in the mole ratio specified by the chemical equation. To do so, you will first have to determine the concentration of the copper ions in your water in moles per liter so you can match that concentration with the appropriate concentration of reactant.
465
Example 2
Precipitation of Calcium Phosphate
Aqueous calcium chloride, CaCl2(aq), reacts with aqueous sodium phosphate, Na3PO4(aq), forming a precipitate of calcium phosphate, Ca3(PO4)2(s).
If 12 mol of CaCl2(aq) react with 8 mol of Na3PO4(aq), will there be any reactant left over? If so, which reactant?
If 12 mol of CaCl2(aq) react with 16 mol of Na3PO4(aq), will there be any reactant left over? If so, which reactant?
If 12 mol of CaCl2(aq) react with 4 mol of Na3PO4(aq), will there be any reactant left over? If so, which reactant?
Solution
First, write a balanced chemical equation for the reaction. Because atoms are conserved, NaCl(aq) must be the other product.
3CaCl2(aq) + 2Na3PO4(aq) → Ca3(PO4)2(s) + 6NaCl(aq)
The mole ratio for CaCl2 and Na3PO4 is 3:2. For any other ratio, there will be leftover reactant.
12 mol CaCl2 to 8 mol Na3PO4 is a ratio of 3:2. So all the reactants will be converted to products.
12 mol CaCl2 to 16 mol Na3PO4 is a ratio of 3:4. There will be some Na3PO4 left over.
12 mol CaCl2 to 4 mol Na3PO4 is a ratio of 4:1. There will be some CaCl2 left over.
LESSON SUMMARY
LESSON SUMMARY
How can you convert all the reactants to products?
KEY TERMS
mole ratio
limiting reactant (limiting reagent)
Coefficients in chemical equations represent the proportions in which moles of reactants combine and products form. The ratio of two coefficients is also called the mole ratio. To produce the maximum amount of product, moles of reactants should be combined in the exact proportions specified by the coefficients. Mass and volume amounts cannot be substituted for coefficients. If there is not enough of a reactant, resulting in other reactants being left over, that reactant is called a limiting reactant.
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
Calcium phosphate is similar to the solid that forms bones and teeth. The calcium cations and phosphate anions dissolved in your blood deposit as solids to form your skeleton.
Exercises
Reading Questions
Why are coefficients important in chemical equations?
Explain how to create the maximum amount of product from a reaction.
466
Reason and Apply
For each of the reactions listed, determine the mole ratio of reactants that produces the maximum amount of precipitate. Be sure to balance the equations.
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
Cu(NO3)2(aq) + K2CO3(aq) → CuCO3(s) + KNO3(aq)
ZnCl2(aq) + NaOH(aq) → Zn(OH)2(s) + NaCl(aq)
CaCl2(aq) + Na2C2O4(aq) → CaC2O4(s) + NaCl(aq)
Lab Report Write a lab report for the experiment you did in which you found the mole ratio by precipitating solids.

Aqueous silver nitrate reacts with aqueous sodium chloride producing a precipitate of silver chloride.
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
How many moles of NaCl do you need to react with 0.10 mol of AgNO3?
How many grams of NaCl does this represent?
Imagine that you have 500 mL of 0.20 M silver nitrate solution. How many milliliters of 0.50 M sodium chloride solution should you add to remove all the silver ions from the solution?