LESSON 95: Not So Hot
486
THINK ABOUT IT

You just received a bad bruise on your leg playing sports. The bruise is swelling and is quite painful. Your coach pulls out a disposable cold pack from the first-aid kit. A quick twist of the package activates it, and the temperature of the cold pack suddenly decreases. It feels nice and cold on your injury. What is the source of this cold sensation?
In what direction is heat transferred during a chemical process?
To answer this question, you will explore
Heat
Exothermic and Endothermic Processes
Kinetic Energy
Heat
EXPLORING THE TOPIC
Heat
Therapeutic hot and cold packs are fairly easy to make. In one type of hot pack, solid calcium chloride, CaCl2, and water are separated into two different pouches. When the pack is twisted, the pouches break open and the solid CaCl2 dissolves in the water, releasing heat. The temperature of the solution increases and the bag feels hot.
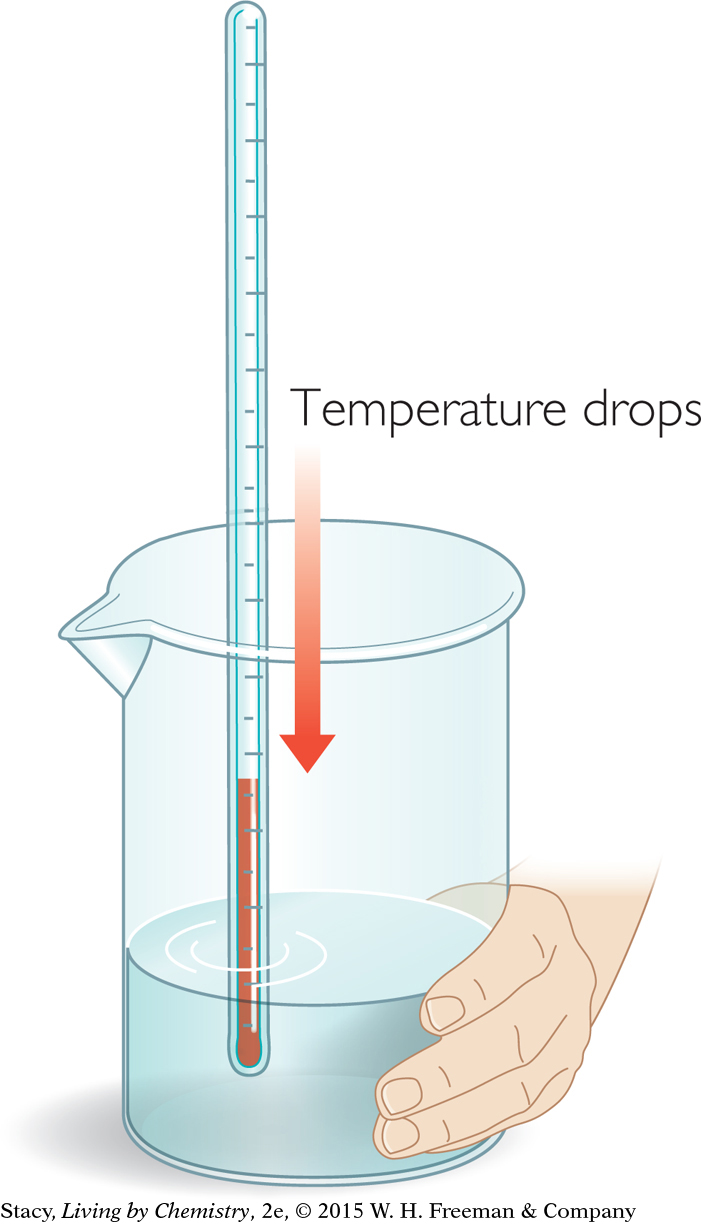
In contrast, in one type of cold pack, solid ammonium nitrate, NH4NO3, and water are separated into two different pouches. When you twist the pack and the NH4NO3 dissolves in the water, the temperature of the solution decreases and the bag feels cold. If you perform these two reactions in beakers, you can measure the temperature changes with a thermometer.
What the thermometer records and your hand experiences is energy transferred into or out of the products of the chemical change. This process of energy transfer is called heat. Heat is a transfer of energy between two objects due to temperature differences. Heat always transfers from a higher temperature to a lower temperature. So, for instance, when the beaker feels cold to your hand, it is because heat transfers from your hand to the beaker.
SYSTEM AND SURROUNDINGS
To communicate clearly about heat transfer, it is necessary to specify where the heat is transferring to or from. The matter that you are focusing on is referred to as the system. Once you have defined the system, everything else is referred to as the surroundings, or environment. If the solution of ammonium nitrate and water is the system, then the beaker, the thermometer, the air around the beaker, your hand, and everything else in the universe constitute the surroundings. Heat transfers between a system and its surroundings.
487
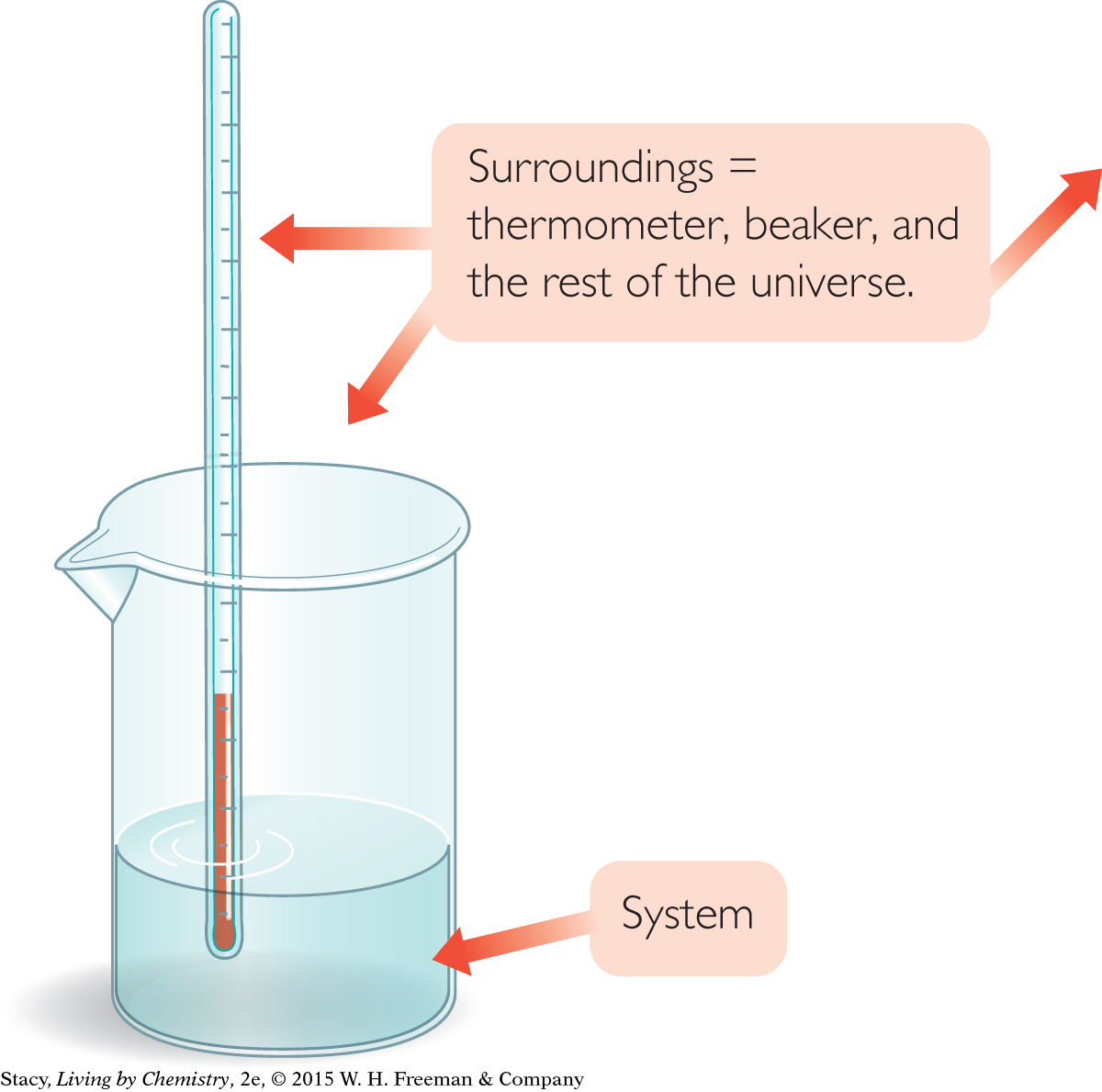
Big Idea
Big Idea
Heat is a transfer of energy due to temperature differences.
Exothermic and Endothermic Processes
Exothermic and Endothermic Processes
CONSUMER CONNECTION
CONSUMER
CONNECTION
Most household refrigerators contain a liquid called a refrigerant that circulates in a series of coiled tubes. As it evaporates, the refrigerant absorbs heat from inside of the refrigerator. The refrigerant is then condensed back into a liquid in tubes located in back of the refrigerator, releasing heat, and the process starts all over again.

Chemists categorize chemical changes according to the direction of heat transfer. As you learned in the previous lesson, when heat is transferred out of the system to the surroundings, the process is exothermic. When heat is transferred from the surroundings to the system, the process is endothermic. (In Greek, exo means outside and endo means inside.) Exothermic processes are experienced as warm by an observer. Endothermic processes are experienced as cold by an observer.
Some examples of exothermic and endothermic changes are listed below. The word heat is included in the chemical equation to highlight the direction of heat transfer.
EXOTHERMIC PROCESSES
Burning methane gas
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) + heat
Dissolving calcium chloride in water
CaCl2(s) → Ca2+(aq) → 2Cl−(aq) + heat
Neutralization of sodium hydroxide with hydrochloric acid
NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq) + heat
ENDOTHERMIC PROCESSES
488
Dissolving ammonium nitrate in water
NH4NO3(s) + heat → NH4+(aq) + NO3−(aq)
Decomposition of mercury oxide
2HgO(l) + heat → 2Hg(l) + O2(g)
Notice that heat transfer is not limited to chemical reactions. The process of dissolving is also exothermic or endothermic depending on the substance. This is because energy is involved every time atoms are rearranged.
Kinetic Energy
Kinetic Energy

Changes in temperature due to a chemical reaction are associated with changes in motion. Kinetic energy is the energy of motion, and temperature is a reflection of the average kinetic energy of a sample. If the products of a reaction are hotter than the reactants, they must be moving faster.
The reaction between H2 and O2 to produce H2O is an extremely exothermic reaction. It is the reaction used to launch the space shuttle. The water molecules produced in the reaction are so hot and they expand so rapidly that the water vapor thrusts the space shuttle up into the air. The photo here provides evidence that the product molecules are moving with explosive speed.
A great deal of the heat energy of this reaction is converted to kinetic energy in the form of rapidly moving water molecules. While the water molecules push the space shuttle up, they transfer some of their kinetic energy to the space shuttle. They also transfer some of their energy to the surroundings as heat. With less kinetic energy, the water molecules gradually become cooler and move more slowly.
LESSON SUMMARY
LESSON SUMMARY
In what direction is heat transferred during a chemical process?
KEY TERMS
heat
system
surroundings
endothermic
kinetic energy
Heat is a transfer of energy between two objects due to temperature differences. Heat transfer accompanies all chemical changes. The heat is transferred either into the system or out of the system. Exothermic reactions are chemical processes that result in the transfer of heat from the products of the reaction (the system) to the surroundings. These reactions feel hot to the observer. Endothermic reactions are chemical processes that result in the transfer of heat from the surroundings to the products of the reaction. These reactions feel cold to the observer. Because temperature is directly related to the motion of molecules, hotter products mean faster moving molecules. Colder products mean slower moving molecules.
489
Exercises
Reading Questions
In your own words, define exothermic and endothermic chemical changes.
Why does an endothermic reaction that takes place in a beaker cause the beaker to feel cold?
Reason and Apply
Methane, CH4(g), reacts with oxygen, O2(g), to produce carbon dioxide, CO2(g), and water, H2O(g). You observe flames.
How does the average kinetic energy of the reactants differ from the average kinetic energy of the products?
Describe what you would expect to see if you had a molecular view of the products and reactants.
You mix solid copper (II) sulfate, CuSO4(s), with a small amount of water, H2O(l) in a beaker. Hydrated copper (II) sulfate, CuSO4 · 5H2O(s) is produced. A thermometer inside the beaker indicates that the temperature has increased from 19 ºC to 48 ºC.
CuSO4(s) + 5H2O(l) → CuSO4 · 5H2O(s)
List all the substances that are part of the system.
List at least four things that are part of the surroundings.
Which is at a lower temperature: CuSO4(s) or CuSO4 · 5H2O(s)? Explain your thinking.
What will you feel if you touch the beaker?
Is the process endothermic or exothermic? Explain your thinking.
What evidence do you have that heat is transferred to the surroundings from the products of the reaction?
You mix solid hydrated barium hydroxide, Ba(OH)2 · 8H2O(s), and solid ammonium nitrate, 2NH4NO3(s), in a beaker. The reaction is shown here. A small pool of water in contact with the outside of the beaker freezes.
Ba(OH)2 · 8H2O(s) + 2NH4NO3(s) → 2NH3(g) + 10H2O(l) + Ba(NO3)2(aq)
List all the substances that are part of the system.
List at least four objects that are part of the surroundings.
Which is at a lower temperature: NH4NO3(s) or Ba(NO3)2(aq)? Explain your thinking.
What will you feel if you touch the beaker?
Is the reaction endothermic or exothermic? Explain your thinking.
What evidence do you have that heat is transferred from the surroundings to the products of the reaction?