LESSON 98: The Heat Is On
500
THINK ABOUT IT

Imagine that you put a metal pan and a pizza in a hot oven. Both the pizza and the pan start out at room temperature. You leave them in the oven for the same amount of time. Will they heat up to the same temperature? And once they are taken out of the oven, will they cool off differently?
How do different substances respond to heat?
To answer this question, you will explore
Specific Heat Capacity
Bonding, Numbers, and Heat
Specific Heat Capacity
EXPLORING THE TOPIC
Specific Heat Capacity
Every substance has a unique response to heat. Suppose that you place an aluminum pot of water on the stove to heat. After a minute, the metal pot will be too hot to touch, but the water will still be cool. This is because different amounts of energy are needed to raise the temperature of each type of substance.
To compare heat transfer to different substances, you can measure the energy required to raise the temperature of the same mass of each substance by the same number of degrees. The amounts of energy needed to raise the temperature of 1 g of several sample substances by 1 ºC are given in the table.
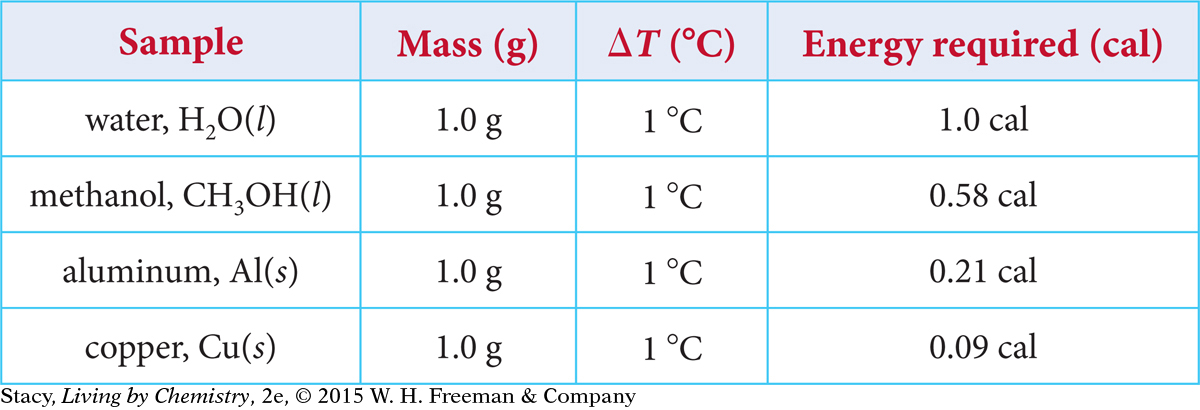
Notice that fewer calories of energy are required to raise the temperature of methanol, aluminum, and copper from 20 ºC to 21 ºC, compared with water. This is consistent with the observation that the aluminum pot heats up and cools off faster than the water in the pot. Less energy is involved in changing the temperature of the aluminum.
501
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
Deserts can be extremely hot at midday and extremely cold at night. The lack of water vapor in the air leads to greater temperature fluctuations.

The heat required to raise the temperature of 1 g of a substance by 1 ºC is called the specific heat capacity. Every substance has a specific heat capacity. Some values are shown in the table.
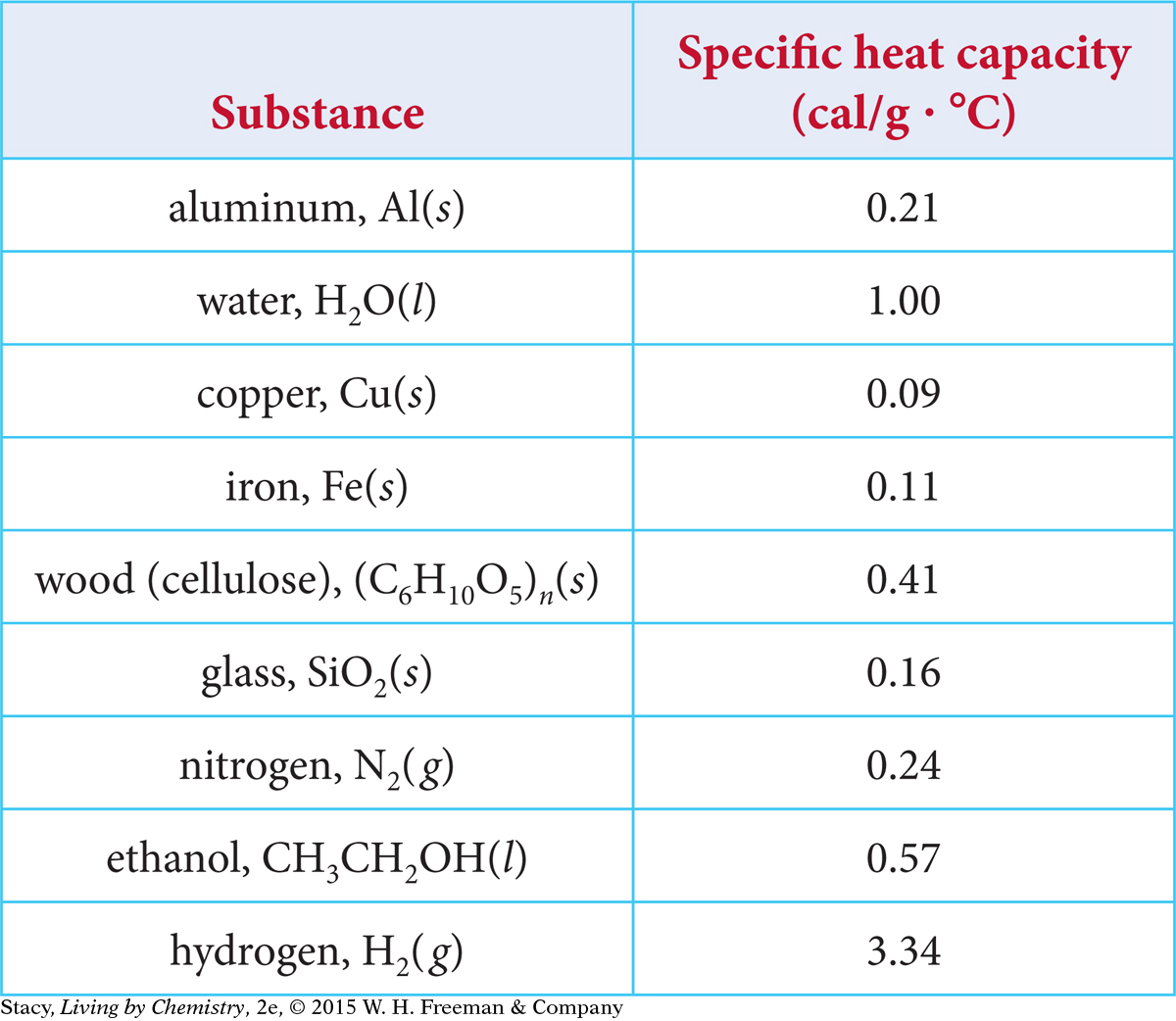
The greater the specific heat capacity of a substance, the less its temperature will rise when it absorbs a given amount of energy. If a substance has a low specific heat capacity, it will change temperature easily, with a small transfer of energy. This applies whether the energy is being transferred into or out of the substance. This is why the aluminum pot heats up faster, and also cools down faster, than water. Water does not change temperature as easily as most substances.
Big Idea
Big Idea
Substances with low specific heat capacities can heat up and cool down easily with only a small transfer of energy.
The specific heat capacity of a substance can be used to determine the amount of energy needed to raise the temperature of any mass by any number of degrees. The formula for heat transfer to any substance is a product of the mass, m, its specific heat capacity, Cp, and the temperature change, ΔT.
q = mCpΔT
Example 1
Cooling
Suppose that you have 15 g of methanol, CH3OH, and 15 g of water, H2O, both at 75 ºC. You want to cool both samples to 20 ºC. How much energy (in calories) do you need to remove from each sample?
Solution
The energy transferred is a product of the mass, the specific heat capacity, and the change in temperature.
q = mCpΔT
502
The mass of each sample is 15 g, and the change in temperature is 20 ºC minus 75 ºC, or −55 ºC. However, the specific heat capacities of water and methanol are different. The specific heat capacity of water is 1 cal/g · ºC. The specific heat capacity of methanol is 0.58 cal/g · ºC.
| Water: | Methanol: |
| q = (15 g)(1 cal/g · ºC)(− 55 ºC) | q = (15 g)(0.58 cal/g · ºC)(− 55 ºC) |
| = −830 cal | = −480 cal |
It takes a greater transfer of energy to cool water than to cool methanol. Because of this, water is a very good insulator and retains its temperature. Note that the negative signs indicate that energy is transferred from the system to the surroundings.
Example 2
Final Temperature
Imagine that you have 3.5 g of copper and 3.5 g of water. Both are at 25 ºC. Which sample will be at a higher temperature if you transfer 150 cal to each? Show your work.
Solution
The energy transferred is a product of the mass, the specific heat capacity, and the change in temperature.
q = mCpΔT
You know the value of q and the masses of the water and copper samples. You want to find the temperature change. Solve for ΔT. You can rearrange the equation:
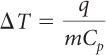
You can look up the specific heat capacities of water and copper in a table like the one on page 501. The specific heat capacity of water is 1 cal/g · ºC. The specific heat capacity of copper is 0.09 cal/g · ºC.
| Water: | Copper: |
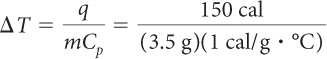
|
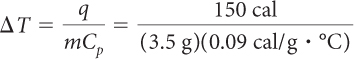
|
| = 43 °C | = 480 °C |
The temperature of copper increases about ten times as much as the temperature of the same quantity of water when 150 calories of energy are transferred.
To find the final temperature, add the temperature change to the initial temperature.
Water: Final temperature = 25°C + 43°C = 68°C
Copper: Final temperature = 25°C + 480°C = 505°C
For the same amount of heat transfer, the copper is much hotter.
Bonding, Numbers, and Heat
Bonding, Numbers, and Heat
503
CONSUMER CONNECTION
CONSUMER
CONNECTION
To make ice cream, the ingredients must be mixed at a temperature below 0 ºC. This is why you add salt to the ice in the outermost chamber of an ice-cream maker. Salt water has a lower freezing point than water without salt. The salt water with ice cubes is at a lower temperature than pure water with ice cubes. Heat is transferred from the ice cream to the salt-ice mixture, lowering the temperature to below 0 ºC.

It may seem strange that different amounts of energy are needed to raise the temperature of different substances. Why would you need more energy for water, compared with the same mass of methanol, aluminum, or copper to heat them all to the same temperature?
The answer is partially related to molar mass. Look back at the table of specific heat capacities on page 501. While the molar mass of aluminum is 27.0 g/mol, the molar mass of copper is 63.6 g/mol. So, there are more atoms in 1 g of aluminum than in 1 g of copper. It makes sense that more energy is required to cause a larger number of atoms to move faster.
Bonding also has an effect on the specific heat capacity of a substance. Substances that are polar tend to have high specific heat capacities. This means that molecules will have higher specific heat capacities than metals.
Finally, complicated molecules with large numbers of atoms have a variety of internal vibrational motions. The atoms within the molecule can vibrate back and forth like balls on a spring. And the entire molecule can rotate. All of these motions need to be increased to raise the temperature. So, substantially more heat is required to increase the average kinetic energy of molecules compared with individual atoms.
HIGH SPECIFIC HEAT CAPACITY OF WATER
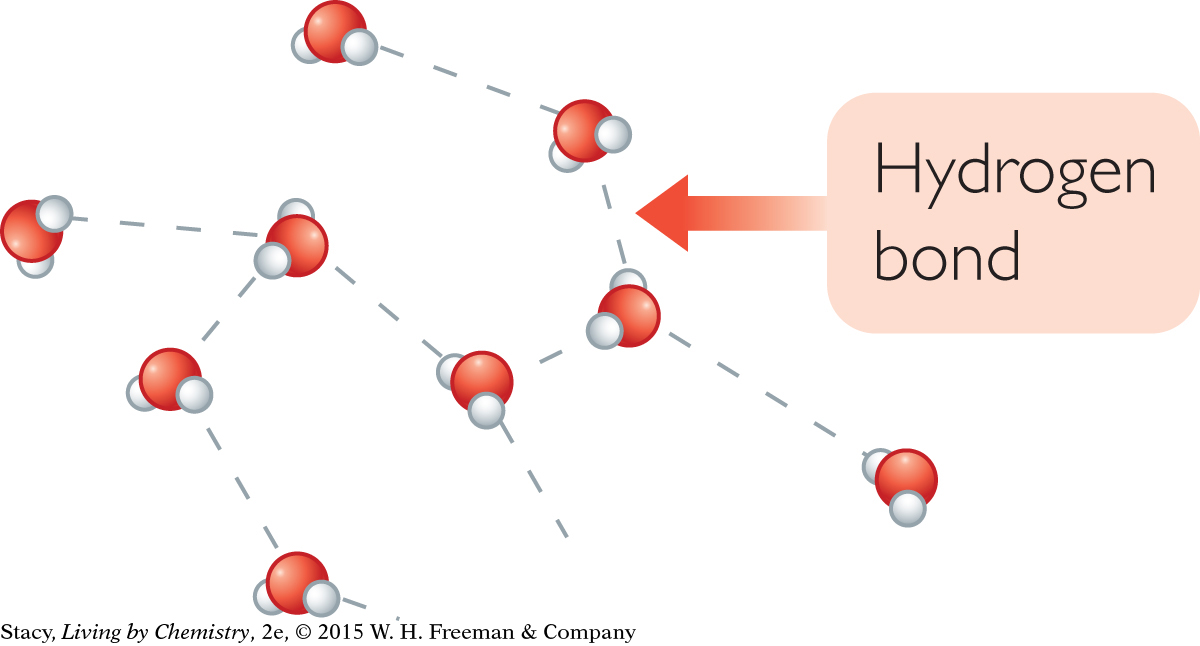
Water has a particularly high specific heat capacity for several reasons. First, the molar mass of water, 18.0 g/mol, is quite small. So there are a lot of water molecules to get moving in a 1 g sample. Second, because water is molecular, it has complex internal movements. Third, water consists of H2O molecules with strong intermolecular attractions called hydrogen bonds. A hydrogen bond is the attraction between the positive and negative dipoles of different water molecules. The hydrogen bonding restricts the motions of the molecules. Extra heat needs to be added to overcome these attractions and raise the kinetic energy. This is why the water in the metal pot heats up so much more slowly than the metal pot itself.
LESSON SUMMARY
LESSON SUMMARY
How do different substances respond to heat?
KEY TERMS
specific heat capacity
hydrogen bond
To increase the temperature of a sample of matter, you must supply energy to it. But the addition of a given amount of energy does not always result in the same rise in temperature. Substances differ in their responses to heat. Some substances, like metals, change temperature more than others in response to the transfer of the same amount of energy. This is because they have a lower specific heat capacity. Specific heat capacity is the amount of energy needed to raise the temperature of 1 g of a substance by 1 ºC. The specific heat capacity of a substance depends on a number of factors, including the number of atoms or molecules in a sample and the type of bonding.
504
Exercises
Reading Questions
What is specific heat capacity? Give the specific heat capacity of a substance, and explain what that means in terms of that substance.
Why is more energy required to raise the temperature of 1.0 g of water compared with 1.0 g of aluminum?
Reason and Apply
Which substance in each pair has the higher specific heat capacity? Justify your choice with an atomic view.
Al or Pb
H2 or Ar
F2 or Cl2
Imagine that you transfer the same amount of energy to all of the substances in the table of specific heat capacities on page 501. Which one would be hottest to the touch? Coolest? Explain your reasoning.
Use specific heat capacity to explain why metal keys left in the sun are hotter than a plastic pair of sunglasses left in the sun.
How much energy is required to raise the temperature of 50 g of methanol from 20 ºC to 70 ºC?
If you place 20 g of aluminum and 20 g of copper, both at 20 ºC, into ice water, which will cool faster? Explain.
Imagine that you have 50 g of water and 50 g of methanol, each in a 100 mL beaker. You place both on a hot plate on low heat.
Which sample will be at a higher temperature after 5 min? Explain your thinking.
If the initial temperature of each liquid is 23 ºC, what is the temperature of each after 25 cal of energy are transferred from the hot plate to each sample?
Would water make a good thermal insulator for a home? Explain your reasoning.
Why do farmers spray water on oranges to protect them from frost? Give two reasons why the water on the orange might help.
