LESSON 101: Now We’re Cooking
516
THINK ABOUT IT
HEALTH CONNECTION
HEALTH
CONNECTION
A healthy diet for the average adult consists of an intake of about 2000 food Calories per day. One fast-food double cheeseburger contains about 750 Calories, a medium soda is about 210 Calories, and a medium order of French fries is 450 Calories. If you ate this for lunch, you would have only 600 Calories left for the rest of the day.

Foods are sources of energy, and certain foods are better sources of energy than others. What are the energy differences between a granola bar, a handful of nuts, and a few potato chips? Burning dry food items is one way to discover the possible energy potential of each type of food.
How are food Calories measured?
To answer this question, you will explore
Foods as Fuel
Calorimetry
Foods as Fuel
EXPLORING THE TOPIC
Foods as Fuel

The foods you eat are sources of energy. There are no fires in your stomach, but your body processes the food you eat to generate energy. Burning two foods and comparing the energy transfer that results is a way to measure the energy of each food.
FOOD CALORIES
Food labels contain a wide variety of nutritional data, including Calorie content, which is a measure of energy. Food Calories, or Calories with a capital C, are kilocalories of energy, abbreviated kcal or Cal. This product label tells you that 1 ounce of potato chips has 160 Cal. In comparison, 1 ounce of walnuts has 190 Cal.
Example
Comparing Calories per Gram
Are there more Calories in 1.0 g of potato chips or 1.0 g of walnuts? (28.3 g = 1.0 oz)
Solution
There are 160 Cal in 1.0 oz of potato chips. There are 190 Cal in 1.0 oz of walnuts. An ounce is the same as 28.3 g.
517
Potato chips: 160 Cal/28.3 g = 5.6 Cal/g
Walnuts: 190 Cal/28.3 g = 6.7 Cal/g
So, there are more Calories available in 1.0 g of walnuts than 1.0 g of potato chips.
According to these calculations, an ounce of walnuts contains more energy than an ounce of potato chips. However, the number of Calories per gram is fairly close. In contrast, raisins contain 3.0 Cal per gram, and raw carrots contain 0.4 Cal per gram.
Calorimetry
Calorimetry
HISTORY CONNECTION
HISTORY
CONNECTION
In the late 18th century, the French scientists Antoine Lavoisier and Pierre Laplace developed an ice calorimeter. With this apparatus, the amount of ice melted by a reaction was measured to determine the energy transferred. A century later, around 1860, the French chemist Marcellin Berthelot constructed the first modern calorimeter.

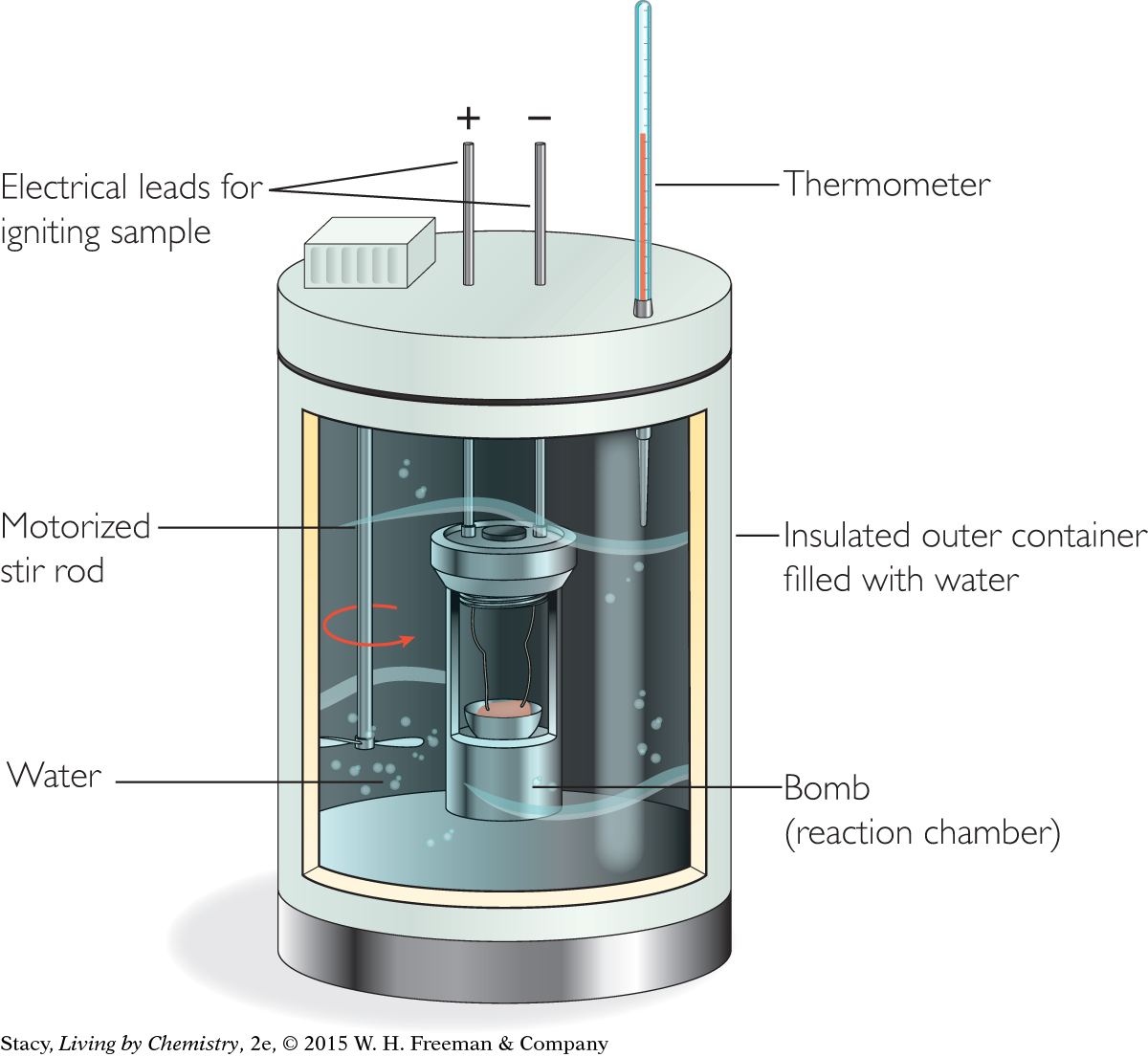
To measure the Calories in food, you have to find out how much energy is released when the food reacts with oxygen. One way to determine the amount of heat transfer during a combustion reaction is to use the reaction between the fuel and oxygen to heat a measured amount of some other substance, such as water. You can then use a thermometer to measure the change in temperature of the water. The science of measuring the energy released or absorbed in a chemical reaction or physical change is called calorimetry. (In Latin, calor means heat, and meter comes from the Greek word for measure.) Chemists and nutritionists alike use a bomb calorimeter to figure out the calorie content of combustible substances.
The illustration shows a bomb calorimeter. The combustion reaction takes place in an inner reaction chamber called a bomb, which is completely surrounded by water. The sample is ignited by electrical energy, and the energy, due to the burning of the fuel, is transferred to the water. A stirring rod makes sure the heat is uniform in the water. If you determine the change in temperature of the water and you know the quantity of water in the calorimeter, you can determine the heat transferred during the combustion reaction.
LESSON SUMMARY
LESSON SUMMARY
518
KEY TERM
calorimetry
How are food Calories measured?
There are a number of different ways to compare the energy transferred when food reacts with oxygen, as it does in the overall digestion process. Some foods can be burned and then their energies can be measured and compared. Calorimetry is the science of measuring the energy released or absorbed by a chemical or physical change. You can use a bomb calorimeter to measure the amount of heat transferred to the surroundings in a combustion reaction.
Exercises
Reading Questions
What do Calories have to do with combustion?
Why does a bomb calorimeter have an inner chamber and an outer chamber?
Reason and Apply
Can you measure the heat energy of a combustion reaction by placing a thermometer directly into the flames? Explain.
What is calorimetry?
Most school laboratories do not have bomb calorimeters.
Design an experiment that would allow you to compare whether a cheese puff snack or a roasted corn snack transfers more energy when burned.
In your experiment, does it matter if you burn the same mass of cheese puff and roasted corn snack? Why or why not?
Why is it important to measure the mass of each snack food at the end of your experiment?
What are some possible sources of error that could occur in your experiment?
Lab Report Write a lab report for the Lab: Calorimetry. In the procedure section, describe your procedure in detail and include a sketch of your setup. In your conclusion, include a discussion of how well your setup worked and whether or not you would change anything.
