LESSON 103: Fuelish Choices
523
THINK ABOUT IT
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
When pure alcohols, such as ethanol and methanol, combust, the flame is almost colorless and therefore dangerous because it is invisible. In 2005, the fuel for the cars racing in the Indianapolis 500 was changed from methanol to ethanol to promote safety. The ethanol now used contains 2% gasoline, which creates a visible yellow-orange flame when it is burning.

Combustion reactions are used to power vehicles, heat water and homes, and create electricity. But not all combustion reactions provide the same amount of energy. Some fuels heat water or power furnaces better than others.
How do different fuels compare?
To answer this question, you will explore
Burning Fuels
Heat of Reaction
Comparing Fuels
Burning Fuels
EXPLORING THE TOPIC
Burning Fuels
There are a number of different choices of fuels to power race cars. Ideally, you want to choose a fuel that transfers the most energy per gram. That way, the fuel does not unnecessarily add to the weight of the car. You can compare the energy from combustion of a variety of fuels using calorimetry to see which one would be best for a race car.
ETHANOL VERSUS METHANOL
Both ethanol, C2H6O, and methanol, CH4O, have been used to power race cars. Suppose that you put the two fuels into alcohol burners and use them to heat two identical 50 mL samples of water by 30 °C.
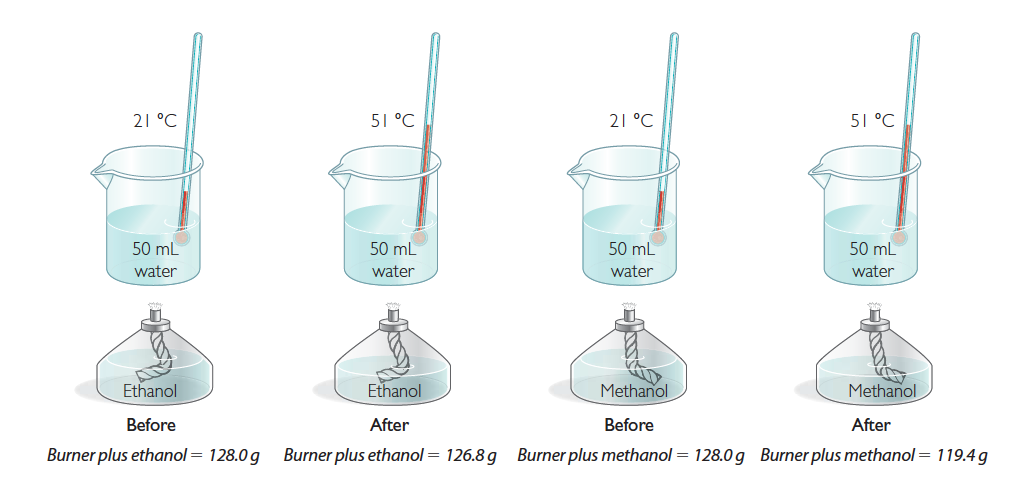
524
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
A biofuel is any plant substance that can be used as a fuel. For example, some people have customized their cars to burn vegetable oils. These people can collect the oil that would have been discarded after deep frying by restaurants for use in their fuel tanks.

The chemical equations for the combustion of each type of fuel are shown here.
Combustion of ethanol: C2H6O(l) + 3O2(g) → 2CO2(g) + 3H2O(l)
Combustion of methanol: 2CH4O(l) + 3O2(g) → 2CO2(g) + 4H2O(l)
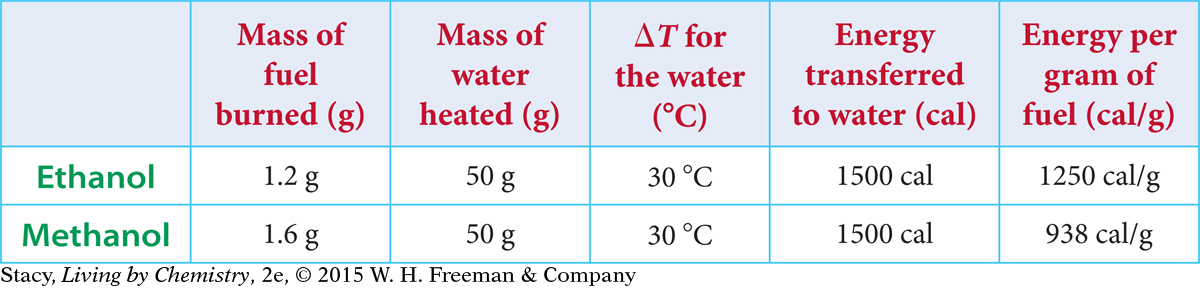
The experimental results show that the combustion of ethanol delivers more energy per gram of fuel consumed, compared with the combustion of methanol. So you would want to use ethanol, not methanol, in a race car if weight were a concern.
Heat of Reaction
Heat of Reaction
The amount of energy transferred during a chemical reaction is called the heat of reaction. It is often designated with the symbol ΔH. The heat of reaction for a combustion reaction is usually referred to as the heat of combustion. Heat of reaction, ΔH, is expressed as a negative number when the reaction is exothermic. This is because energy is transferred from the system. When a reaction is endothermic, the system gains energy. Heat of reaction, ΔH, is expressed as a positive number when the reaction is endothermic.
Scientists have done carefully controlled experiments to measure the heat of combustion for many different fuels. Accurate measurements for the combustion of methanol result in a heat of reaction that is much larger in magnitude than the experimental value reported earlier.
Combustion of methanol: 2CH4O + 3O2 → 2CO2 + 4H2O
ΔH = –5400 cal/g
Important to Know
The heat of combustion is reported with a negative sign to indicate that energy is released by the reaction.
Combustion of ethanol: C2H6O(l) + 3O2(g) → 2CO2(g)+3H2O(l)
ΔH = –7100 cal/g
UNITS
The metric unit that scientists use to express energy is the joule, J. A joule is a unit of energy that is 4.184 times larger than a calorie. The heat of reaction can be expressed in kilocalories per gram, kilocalories per mole, kilojoules per gram, or kilojoules per mole.
1 joule = 0.239 calorie
1 calorie = 4.184 joules
Comparing Fuels
Comparing Fuels
525
One way to compare fuels is to calculate the number of kilojoules of energy per mole (kJ/mol) of fuel. Simply multiply the kilojoules per gram (kJ/g) by the molar mass (g/mol) of each fuel.
This table provides the heats of combustion for several fuels in both kilojoules per gram and kilojoules per mole.
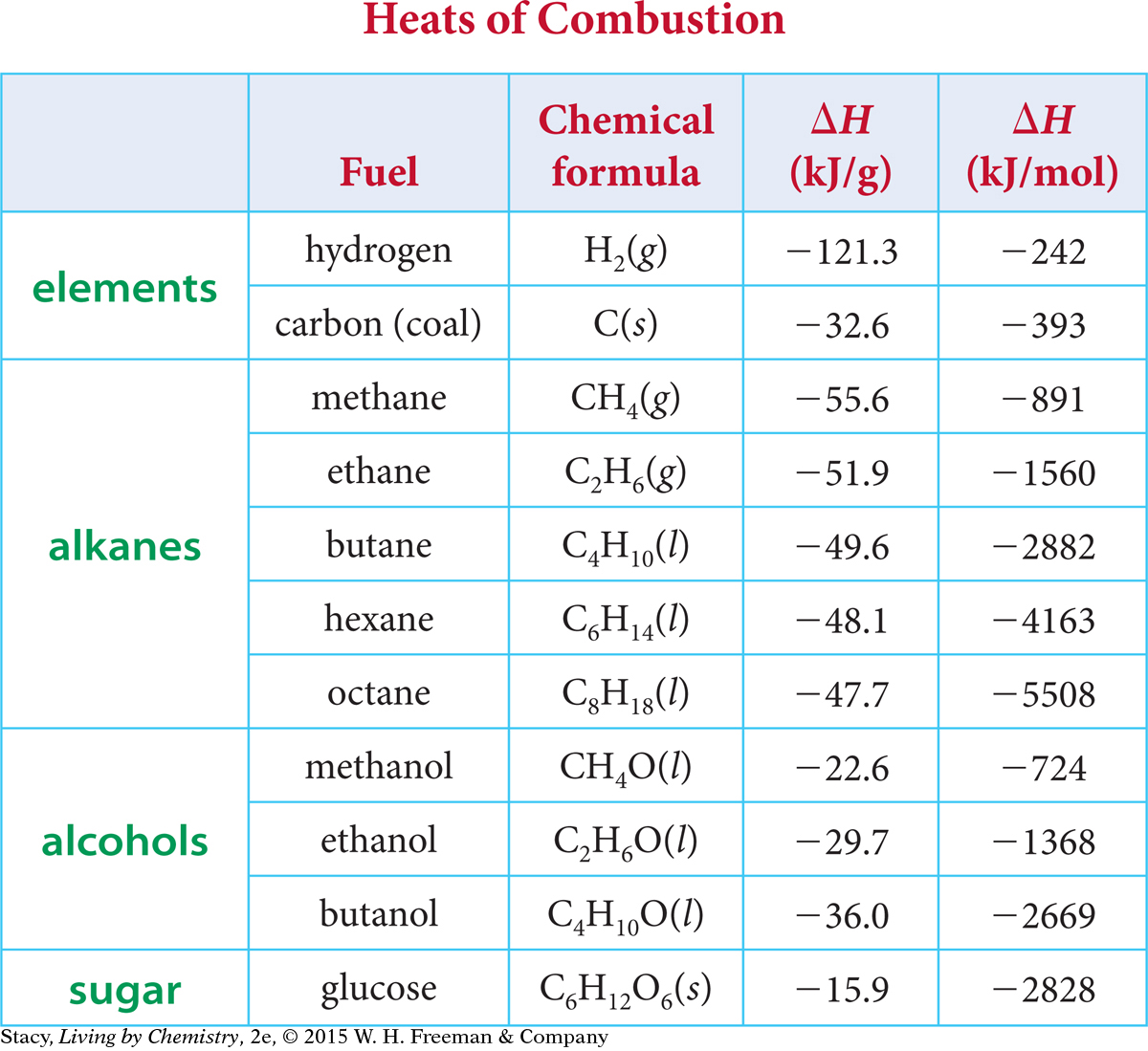
Notice that when the number of carbon atoms increases, the energy per mole increases. Also, the energy transferred per mole is smaller for the alcohols compared with the alkanes. This makes sense because combustion is a reaction in which a substance combines with oxygen, and the alcohols already contain an oxygen atom. Also notice that elemental hydrogen releases the highest energy per gram. This is why hydrogen is used as the fuel to launch a space shuttle. You get a lot of energy without adding much weight.
LESSON SUMMARY
LESSON SUMMARY
How do different fuels compare?
KEY TERMS
heat of reaction
heat of combustion
joule
One way to compare different fuels is to measure the amount of energy they transfer as a result of combustion. Calorimetry is the most common method of measuring this energy. The energy transferred can be expressed in kcal/mol, kcal/g, kJ/mol, or kJ/g of substance. The amount of energy transferred per gram or per mole during a chemical change is commonly called the heat of reaction, ΔH. For combustion reactions, the heat of reaction is negative and is also referred to as the heat of combustion.
Exercises
Reading Questions
526
How can you use water to compare two fuels?
What is heat of combustion?
Explain why heat of combustion is expressed as a negative number.
Reason and Apply
The combustion of 4.0 g of propanol transfers energy to 150 mL of water. The temperature of the water rises from 20 °C to 45 °C.
How many calories of energy are transferred to the water?
How many kilocalories of energy are transferred to the water?
How many kilojoules of energy are transferred to the water?
What temperature increase do you expect for 300 mL of water?
What temperature increase do you expect for 2.0 g of propanol and 150 mL of water?
Write balanced chemical equations for the combustion of the fuels in the heats of combustion table on page 525. What generalization can you make about the amount of oxygen required for combustion of hydrocarbons?
Explain why the combustion of glucose provides a large energy per mole but a rather small energy per gram.
In the heats of combustion table, hydrogen has the highest energy per gram of fuel that is combusted. Research to find three reasons why it is challenging to use hydrogen as a fuel for your automobile.