LESSON 5: All That Glitters: Density
17
THINK ABOUT IT

Gold and lead are metals that are soft and bendable. They are easy to tell apart at a glance because lead is dull gray. But suppose someone tried to trick you by coating a block of lead with a thin layer of gold. How could you prove that this block was a fake?
How can you use mass and volume to determine the identity of a substance?
To answer this question, you will explore
The Definition of Density
Calculating Density
Identifying Matter Using Density
The Definition of Density
EXPLORING THE TOPIC
The Definition of Density

Which is heavier, gold or copper? It depends on the amount of each that you have. If you compare a tiny gold earring and a large copper pipe, the copper will be heavier. If you compare the same volume of each, gold is always heavier than copper. That is because there is more matter in 1.0 cm3 of gold than in 1.0 cm3 of copper. You could also say that gold is denser than copper. The mass of a substance per unit of volume is called its density.
Imagine comparing 1.0 cm3 cubes of wood, water, and copper from larger samples of each material. All three cubes have the same volume, but each cube has a different mass and a different density.
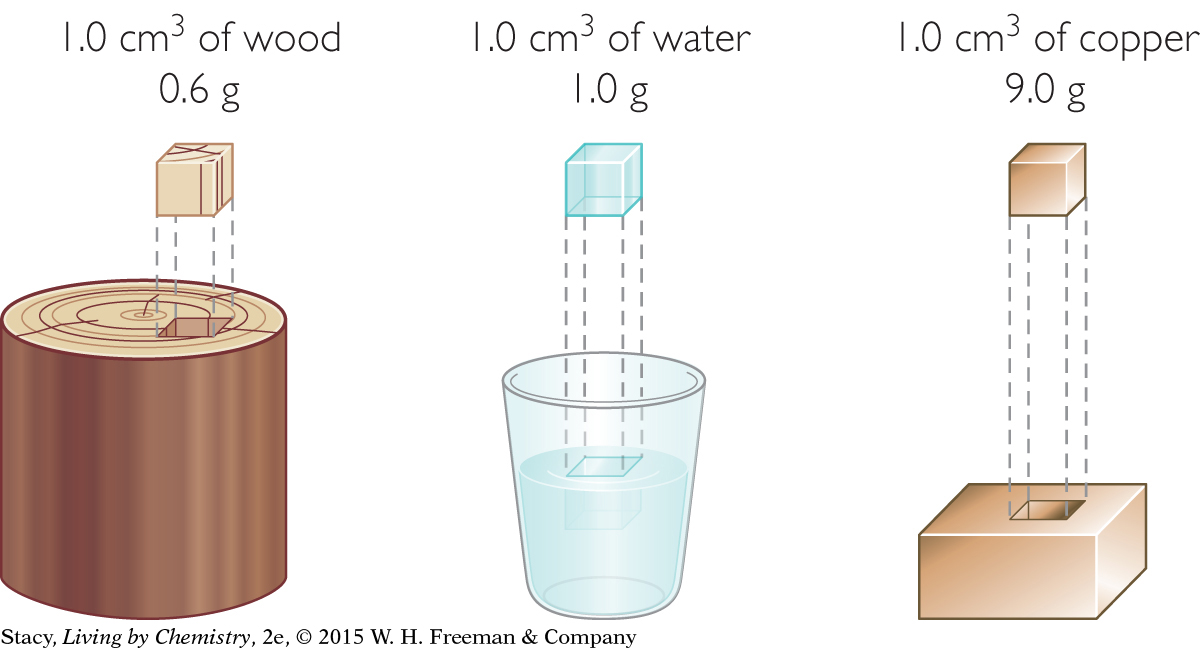
18
Example 1
Differences in Density
Consider the objects on balances shown in these illustrations. How can differences in density account for what you observe in these pictures?
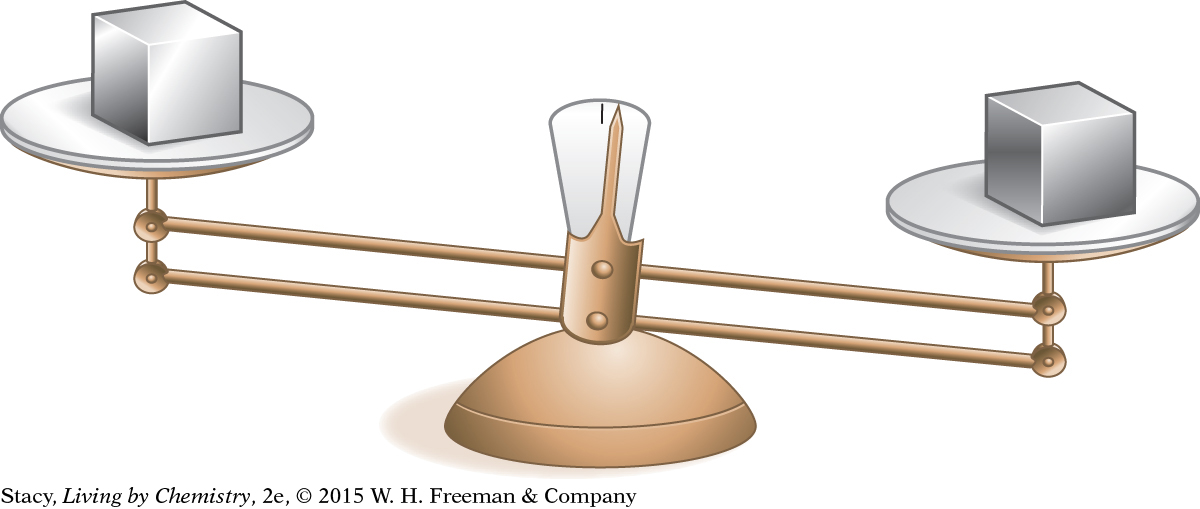
The balance is uneven. The two solid cubes have the same volume but different masses.
|
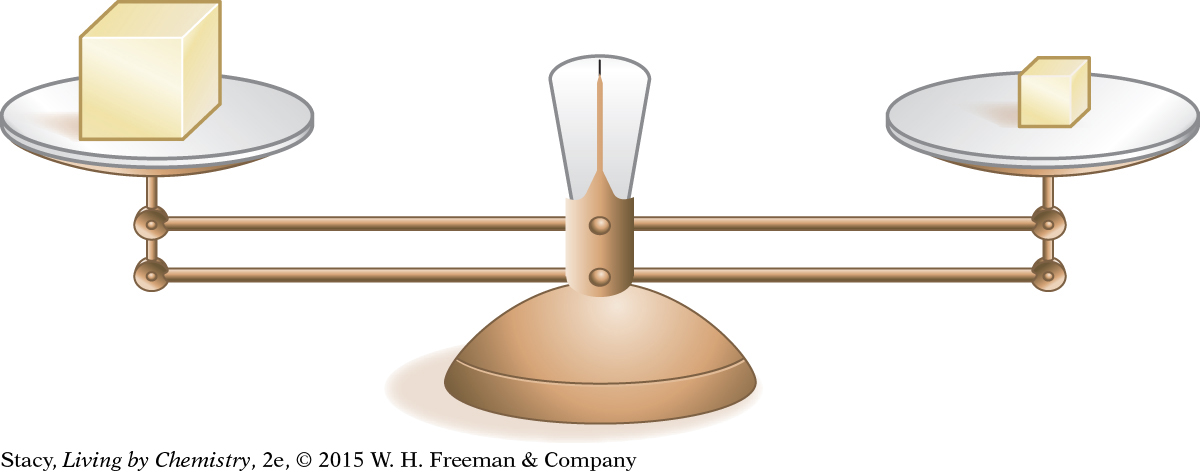
The balance is level. The two solid cubes have the same mass but different volumes.
|
Solution
Because the cubes on the first balance are exactly the same size, for the balance to be uneven one cube must be denser than the other. So the two cubes are made of different materials.
On the second balance, the larger cube must have a lower density than the smaller cube. Again, the two cubes are made of different materials.
Calculating Density
Calculating Density
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Most substances will expand when temperature increases and contract when temperatures decreases. For this reason, there are special joints built in between sections of sidewalks, bridges, railroad tracks, and other structures to prevent cracking or breaking when the volume of the materials change due to temperature.

You can determine the density of a substance without actually cutting out a cubic centimeter sample. Suppose a large chunk of gold has a mass of 309 g and a volume of 16.0 cm3. Divide the mass of the gold by its volume to find its mass per cubic centimeter. This is equal to the density.
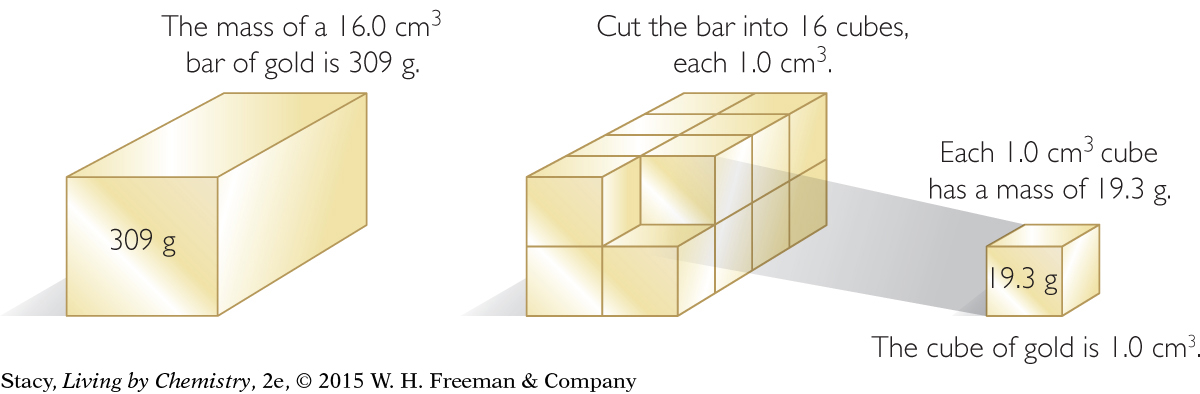
309 g ÷ 16.0 cm3 = 19.3 g/cm3
The density of gold is 19.3 grams per cubic centimeter, or 19.3 g/cm3. This answer is rounded to three significant digits. [For review of this math topic, see MATH Spotlight: Accuracy, Precision, and Significant Digits on page A-1.]
Density
The mathematical formula for density is
where D is the density, m is the mass, and V is the volume.
19
Example 2
Density of a Gold Ring

Suppose you have a gold ring that weighs 7.50 g and has a volume of 0.388 mL. Does it have the same density as a big piece of gold?
Solution
Use the formula to find the density of the ring.
Start with the formula. 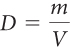
Substitute the values of m and V. 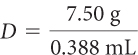
Solve for D. = 19.3 g/ml = 19.3 g/cm3
The ring has the same density as any other sample of gold, big or small. The density of a material does not depend on the shape or size of the sample.
Identifying Matter Using Density
Identifying Matter Using Density
Every substance has certain properties, such as color, hardness, melting point temperature, and density, that depend on the type of matter, not on the amount or size of the sample. These properties are called intensive properties. Intensive properties do not change if the quantity of the substance changes. Therefore, they can be used to help identify that substance.
On the other hand, extensive properties, such as mass or volume, do change depending on the amount of matter. Extensive properties alone can’t be used to help identify a type of matter. For example, knowing that a metal earring has a mass of 3 g doesn’t help you identify what the earring is made of.
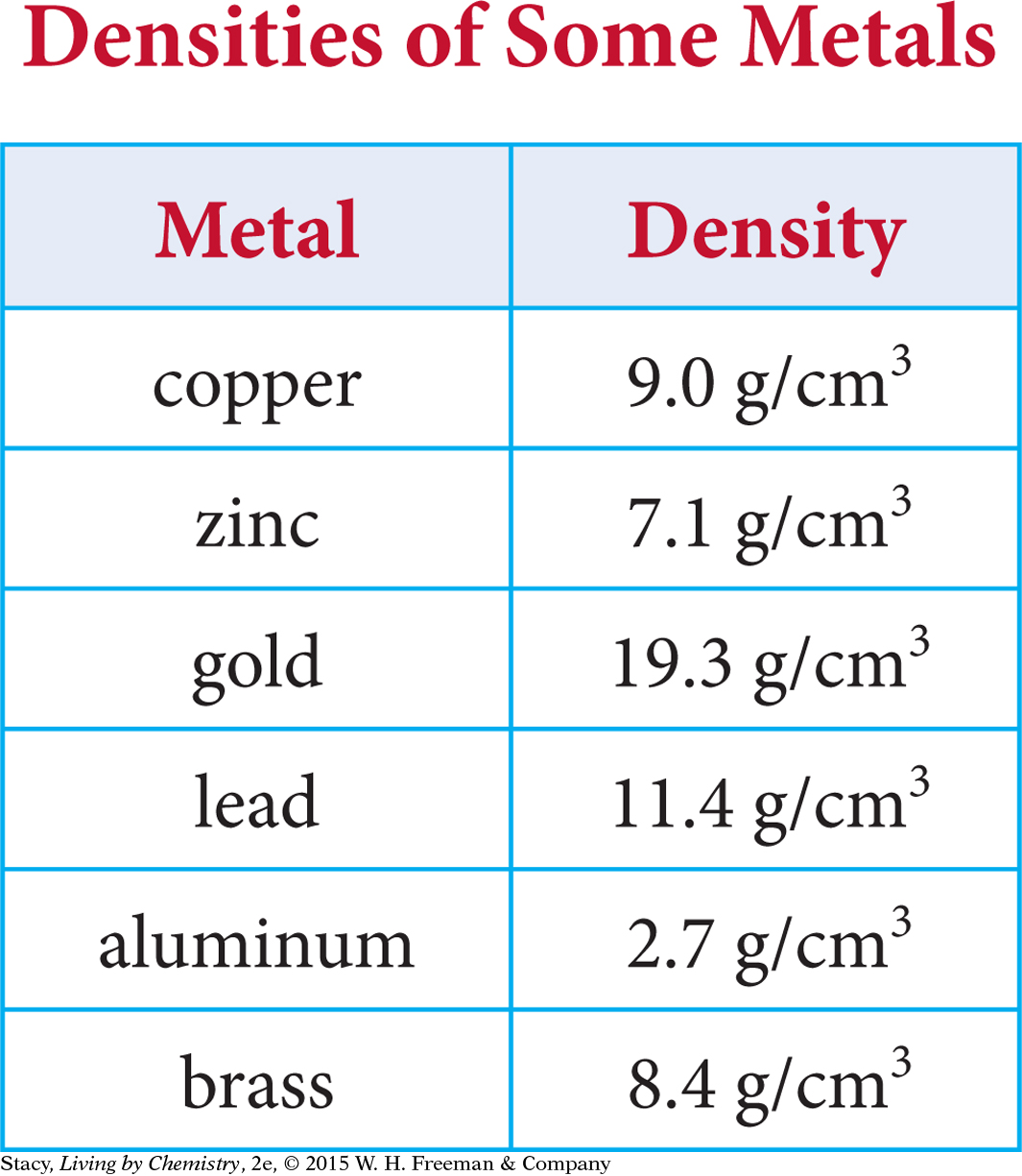
Because density is an intensive property, it can be used to help identify the type of matter that an object or sample is made of. First, determine the density of the object and then compare it with known density values in a reference table like this one to help identify the type of matter.
Big Idea
Big Idea
Each specific type of substance has a particular density. You can identify a substance by its intensive properties, including density.
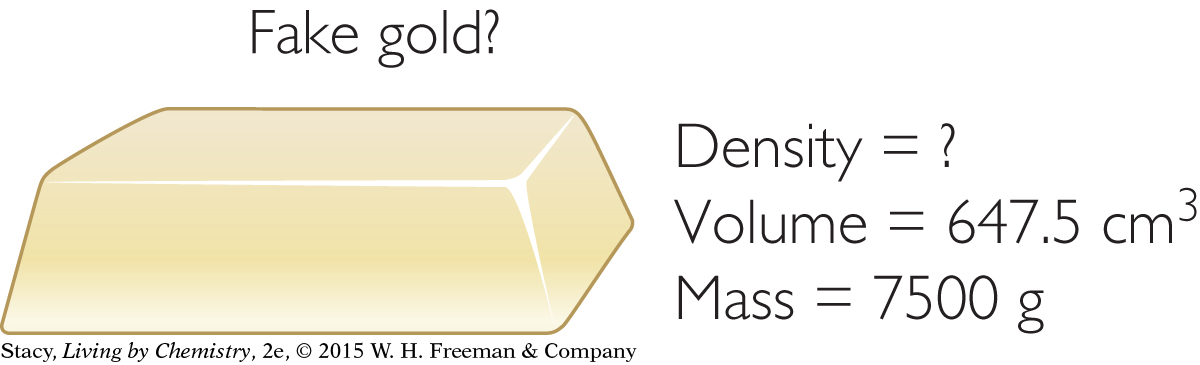
THE FAKE BAR OF GOLD
Important to Know
The density of a substance does not change with its size or its shape. A solid gold bracelet will have the same density as a solid gold brick, and the density of one penny is the same as the density of two pennies.
If someone tried to trick you by coating a block of lead with a thin layer of gold, how could you prove the bar is a fake? One approach would be to scratch the bar to reveal that the inside isn’t gold. Another method would be to use density to prove that the bar isn’t gold.
A block of pure gold will have a density of 19.3 g/cm3.
LESSON SUMMARY
LESSON SUMMARY
20
How can you use mass and volume to determine the identity of a substance?
KEY TERMS
density
intensive property
extensive property
To identify substances, you examine their intensive properties, qualities that do not depend on size or amount. Intensive properties include color, hardness, and density. Density is the mass of a substance per unit of volume. If two substances have different densities, then they are probably made of different types of matter.
Exercises
Reading Questions
In your own words, define density.
Explain how density can be used to determine if the golden penny is made of solid gold.
Reason and Apply
How does the density of aluminum compare with the density of gold? What does this tell you about the amount of matter within each?
If two objects have the same mass, what must be true? Choose the correct answer(s).
They have the same volume.
They are made of the same material.
They contain the same amount of matter.
They have the same density.
Two objects each have a mass of 5.0 g. One has a density of 2.7 g/cm3 and the other has a density of 8.4 g/cm3. Which object has a larger volume? Explain your thinking.
A piece of metal has a volume of 30.0 cm3 and a mass of 252 g. What is its density? What metal do you think this is?
A glass marble has a mass of 18.5 g and a volume of 6.45 cm3.
Determine the density of the marble.
What is the mass of six of these marbles? What is the volume? What is the density?
How does the density of one marble compare with the density of six of the marbles?