LESSON 104: Make It or Break It
530
THINK ABOUT IT
In a chemical reaction, bonds in the reactants are broken and the atoms rearrange to form new bonds in the products. Flames associated with a combustion reaction indicate that energy is released in the form of heat and light. All combustion reactions are exothermic.
Where does the energy from an exothermic reaction come from?
To answer this question, you will explore
Bond Breaking and Bond Making
Energy of Change
Energy Exchange Diagrams
Bond Breaking and Bond Making
EXPLORING THE TOPIC
Bond Breaking and Bond Making
In chemical compounds, atoms are bonded together. When the compound methane burns, it combines with oxygen to make new products. To make carbon dioxide and water from molecules of methane and oxygen, the atoms must rearrange as shown. This means that covalent bonds in the methane and oxygen molecules must be broken and new covalent bonds must be formed.
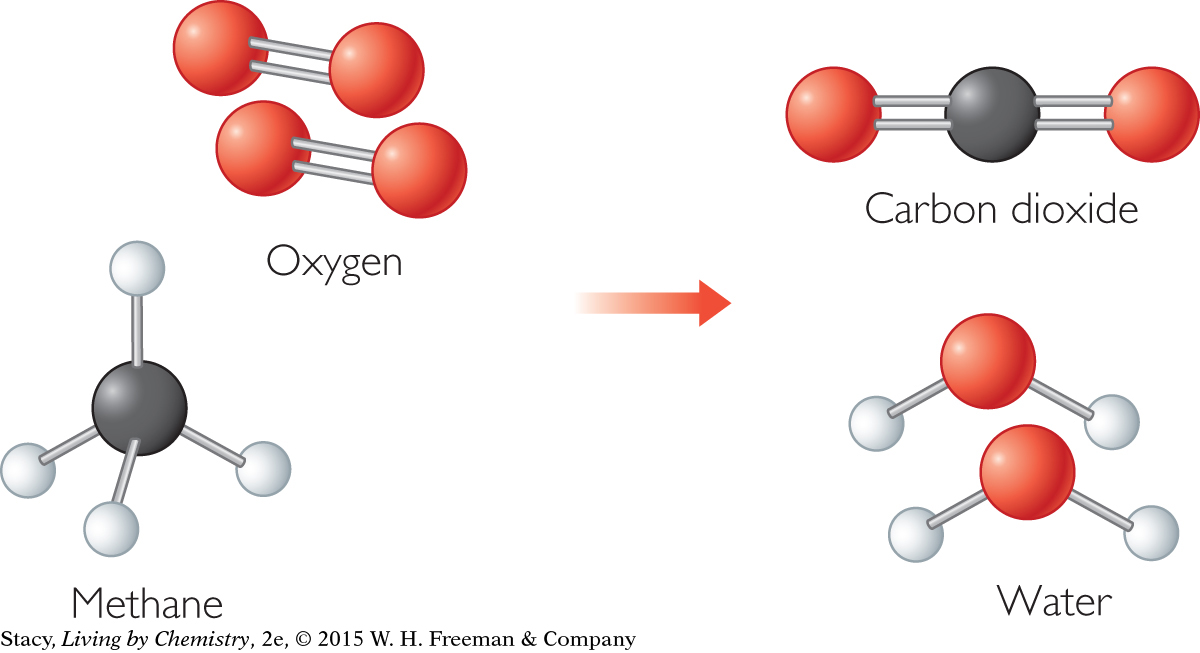
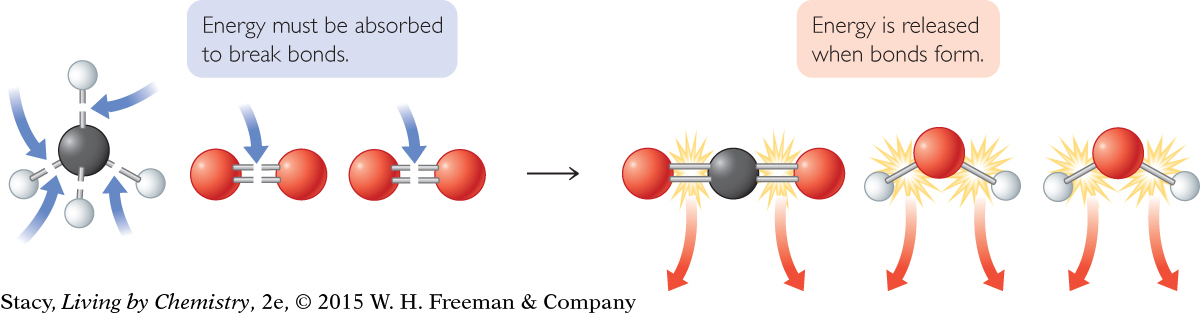
Energy is required to break bonds. Atoms that are bonded together are attracted to each other, so it takes effort to get these atoms apart. So bond breaking is an endothermic process.
In contrast, when new bonds are formed, energy is released. In terms of energy, bond making is the opposite of bond breaking. Because energy is conserved, the amount of energy released when a bond is made is assumed to be equal to the amount of energy required to break that same bond.
531
During a combustion reaction, energy is required to break the bonds in the reactant molecules and energy is released when the bonds form in the product molecules. The net energy released is observed as heat and light.
Big Idea
Big Idea
Bond breaking requires energy. Bond making releases energy.
BOND ENERGY
The amount of energy it takes to break a specific bond, such as a carbon-hydrogen bond, is called the bond energy. Bond energy is considered a measure of the strength of that bond. However, not all C—H bonds have the same bond energy. For example, it takes 435 kJ/mol to break the first C—H bond in a methane molecule, as compared to 339 kJ/mol to break the last C—H bond.
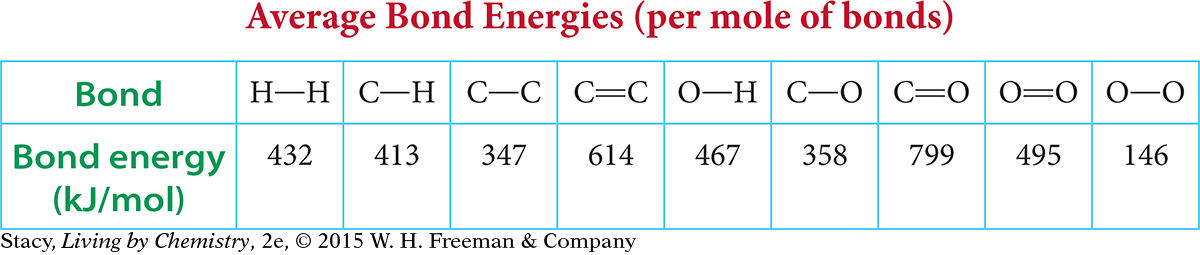
For this reason, bond energies are reported as averages. The bond energy of an average carbon-hydrogen bond is approximately 413 kJ/mol (or 99 kcal/mol). This means that it takes 413 kilojoules of energy to break one mole of carbon-hydrogen bonds.
This table shows the bond energies of some common bonds.
Example
Methane Molecule
How much energy would it take to break all the bonds in 1 mol of methane molecules?
Solution
Each molecule of methane has four C—H bonds. Therefore, each mole of methane molecules has 4 mol of carbon-hydrogen bonds.
413 kJ/mol · 4 mol C—H bonds = 1652 kJ
In reality, all of the bonds of the reactants do not necessarily break to form the products in a chemical reaction. However, this is a useful model to explain energy exchanges related to a chemical reaction.
Energy of Change
Energy of Change
532
To estimate the energy of an entire chemical reaction, you can consider the reaction as if it takes place in two parts—energy in for bond breaking and energy out for bond making.
COMBUSTION OF METHANE
Consider the reaction for the combustion of 1 mol of methane molecules with 2 mol of oxygen molecules. What is the net energy exchange?
CH4 + 2O2 → CO2 + 2H2O
First, figure out how much energy is required to break all the bonds in the reactants.
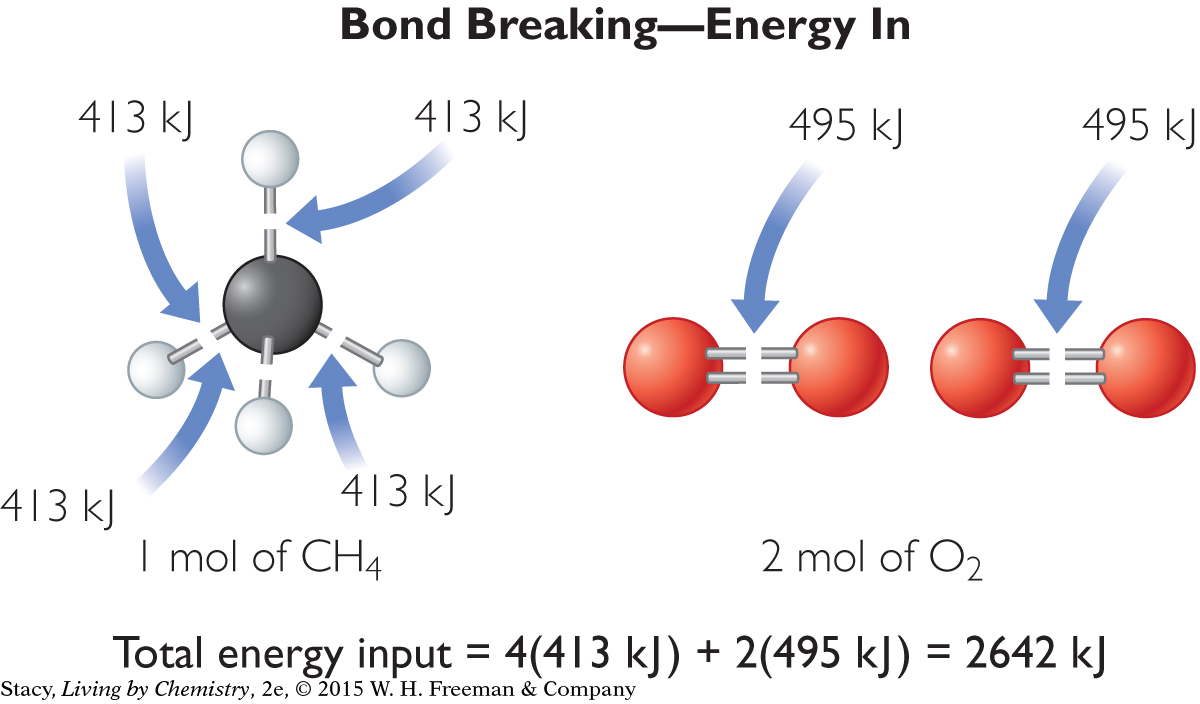
Notice that the energy required to break the bonds of the reactants is positive because energy is added to the system from the surroundings. Now figure out how much energy is released by the formation of new bonds.
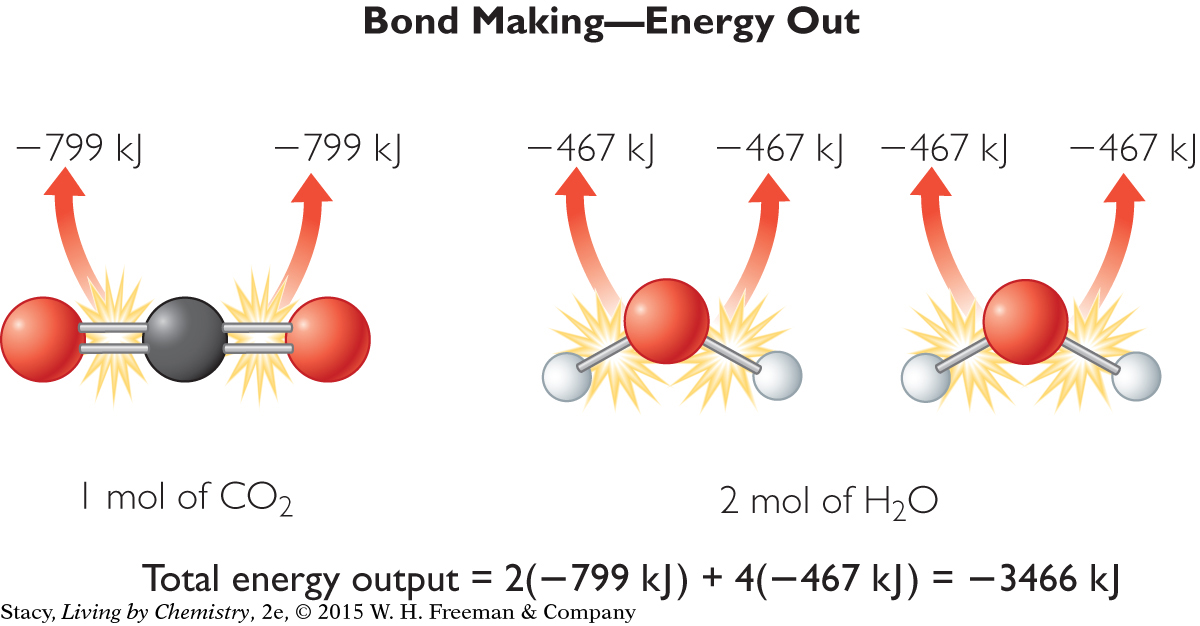
Notice that the energy required to make the bonds of the reactants is negative because energy leaves the system and goes to the surroundings. The net energy exchange is equal to the sum of the energy input and the energy output.
Net energy exchange = (2642 kJ) + (–3466 kJ)
= –824 kJ
The magnitude of energy out is greater than the magnitude of energy in, so this reaction is exothermic. Net energy is expressed as a negative number, because the system loses energy to the surroundings. This value is very close to the actual value for the heat of combustion, ΔH, for this reaction.
Energy Exchange Diagrams
Energy Exchange Diagrams
533
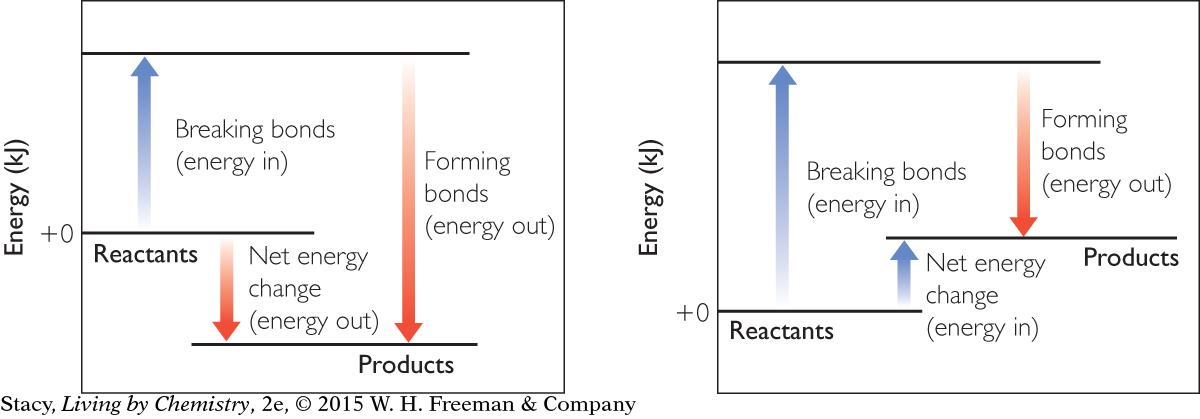
An energy exchange diagram is a way to keep track of the energy changes during a chemical reaction. The arrows pointing up represent the energy going into the system to break the bonds. The arrows pointing down represent the energy released when new bonds are made. The net energy change determines if a reaction is exothermic or endothermic.
LESSON SUMMARY
LESSON SUMMARY
Where does the energy from an exothermic reaction come from?
KEY TERM
bond energy
When reactions take place, bonds between atoms are broken and new bonds are formed. Energy must be transferred to a reactant to break a bond. The average energy required to break a certain type of bond is called its bond energy. Conversely, when new bonds are made, energy is released. The heat transferred during a chemical reaction is equal to the difference in energy between the bond-breaking process and the bond-making process. An energy diagram can be used to keep track of energy exchanges in a chemical reaction.
Exercises
Reading Questions
How can you determine the net energy exchange of a chemical reaction using average bond energies?
Use the energies involved in bond breaking and bond making to explain why combustion reactions are exothermic.
Reason and Apply
Place these bonds in order of increasing energy required to break the bond: H—H, C—C, C=C, O—O, O=O.
Place these bonds in order of increasing energy released when the bond is formed: H—H, C—H, O—H, C=O.
What is the total energy required to break all the bonds in 1 mol of ethanol, C2H6O?
534
Consider the combustion of hydrogen. Use the table of average bond energies to answer the questions.
2H2(g) + O2(g) → 2H2O(l)
What is the total energy required to break the bonds in the reactant molecules?
What is the total energy released by the formation of the bonds in the product molecules?
Use the values from (a) and (b) to determine if this reaction is exothermic or endothermic.
Consider the combustion of methanol and butanol. Use the table of average bond energies to answer these questions.
Methanol: 2CH4O(l) + 3O2(g) → 2CO2(g) + 4H2O(l)
Butanol: C4H10O(l) + 6O2(g) → 4CO2(g) + 5H2O(l)
Calculate the net energy exchange for each reaction.
Compare your answers to the heat of combustion values for methanol and butanol in Lesson 103: Fuelish Choices. What did you discover?
Calculate the net energy change for this reaction:
C2H6(g) → C2H4(g) + H2(g)
In exothermic reactions,
It takes more energy to make all the new bonds in the products than to break all the bonds in the reactants.
There are more bonds made than broken.
There are more bonds broken than made.
It takes more energy to break all the bonds in the reactants than to make all the new bonds in the products.