LESSON 105: Over the Hill
535
THINK ABOUT IT
So far, you have been shown chemical reactions that proceed in one direction. This means that reactants are converted to products until the reaction is complete. Some reactions can be reversed. If a reaction is run in the reverse direction, the products of the reaction are converted back into reactants.
What is the energy associated with reversing reactions?
To answer this question, you will explore
Reversing Reactions
Kinetic and Potential Energy
Activation Energy
Reversing Reactions
EXPLORING THE TOPIC
Reversing Reactions
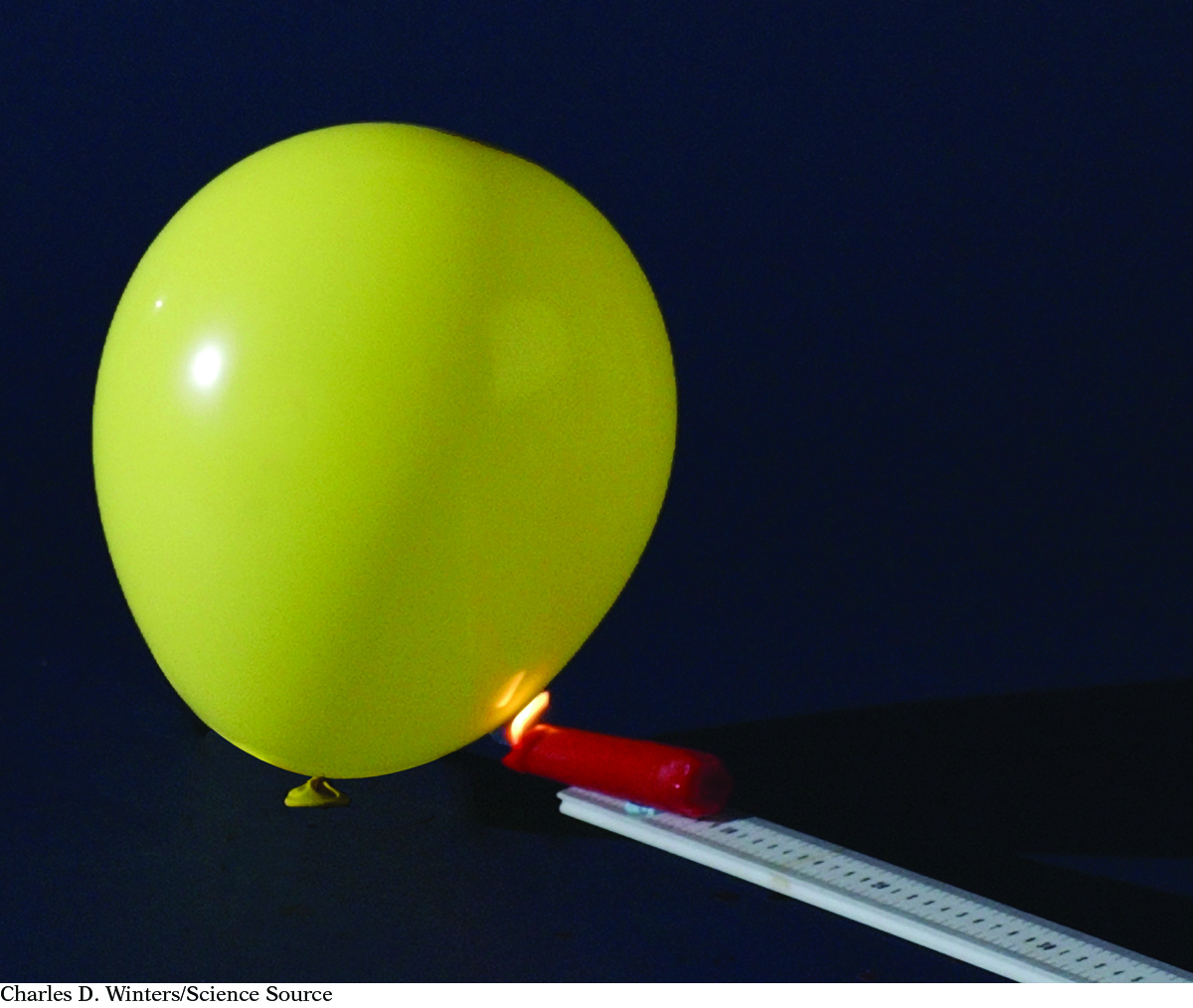
If you place a lit candle under a balloon filled with hydrogen, you can observe the reaction between hydrogen and oxygen. When hydrogen combines with oxygen to form water there is a large “bang.”

2H2(g) + O2(g) → 2H2O(g) ΔH = –482 kJ
The energy released by this reaction is the heat of reaction, –482 kJ. This energy corresponds to 2 mol of H2(g) reacting with 1 mol of O2(g) to form 2 mol of H2O(g). This converts to –241 kJ per mole of H2(g), the value given in the heat of combustion table in Lesson 103: Fuelish Choices.

Important to Know
Heat of reaction values are determined experimentally by calorimetry. You can use average bond energies to estimate the heat of reaction.
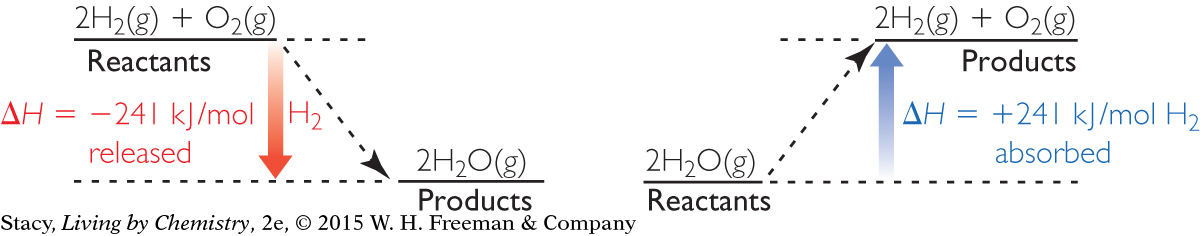
Notice that the decomposition of water into molecules of hydrogen and oxygen is the reverse of the combustion of hydrogen to form water. What is the heat of reaction for this change?
2H2O(g) → 2H2(g) + O2(g) ΔH = +482 kJ
The first law of thermodynamics states that energy is conserved. So, the energy exchange of the reaction in the forward direction is equal and opposite to the energy exchange required for the reverse of the reaction. Notice that when the forward reaction is exothermic, the reverse reaction must be endothermic.
536
Diagrams showing the energy exchange for the formation of water and the decomposition of water are provided here. These energy diagrams are similar to the ones in Lesson 104: Make It or Break It.
Kinetic and Potential Energy
Kinetic and Potential Energy
The energy in a system is a combination of kinetic energy and potential energy. Kinetic energy is the energy of motion. Potential energy is energy that is stored within a physical system and can be converted into another type of energy, such as kinetic energy.
Potential energy can also be defined as the energy associated with the composition of a substance or the position or location of an object in space. For example, a ball at the top of a hill has high potential energy. This potential energy is converted into kinetic energy if the ball rolls down the hill. Likewise, some molecules have a high potential energy. For an exothermic reaction, this potential energy is converted into kinetic energy by converting reactants into products.
CONSERVING ENERGY
These diagrams show the changes in potential energy for an exothermic reaction and an endothermic reaction.
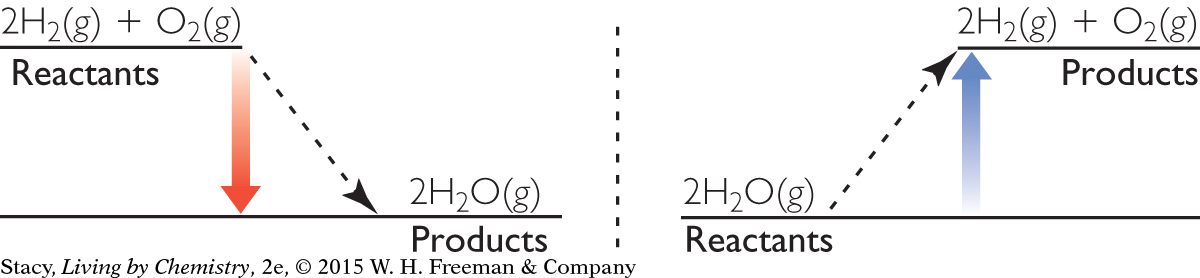
For an exothermic reaction, the potential energy of the system decreases in converting from reactants to products. Because energy is conserved, the sum of the kinetic energy and the potential energy must remain the same, so the kinetic energy increases. The products are hotter than the reactants, because they have a higher average kinetic energy. To reach thermal equilibrium with the surroundings, the hot products transfer energy to the surroundings.
For an endothermic reaction, the potential energy of the system increases. This means that kinetic energy must be converted into potential energy to conserve the total energy. The products are colder than the reactants because they have a lower kinetic energy. To reach thermal equilibrium with the surroundings, energy is transferred from the surroundings to the cold products.
Activation Energy
Activation Energy
537
GEOLOGY CONNECTION
GEOLOGY
CONNECTION
The hydrocarbons that make up Earth’s petroleum reserves are often referred to as “fossil fuels.” This is because much of the oil beneath Earth’s surface was formed from decayed plant and animal remains that were buried deep underground millions of years ago.

A fire can be started with a match. The combustion reaction in an automobile engine is started with a spark plug. All chemical reactions, not just combustion reactions, require some energy input to get started. This is called the activation energy.
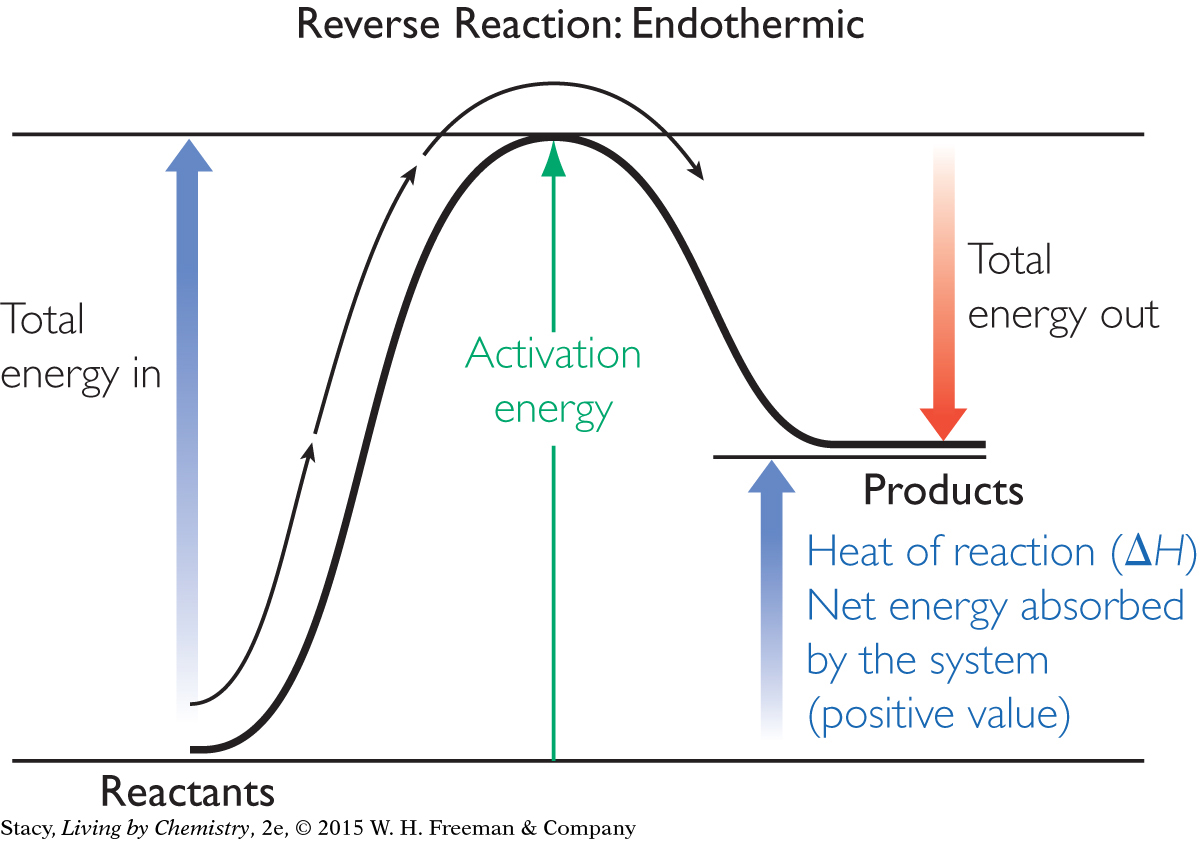
Examine the energy diagram for an exothermic reaction. The potential energy of the products is lower than that of the reactants. However, notice that the potential energy first increases on the pathway from reactants to products before it is converted to kinetic energy.
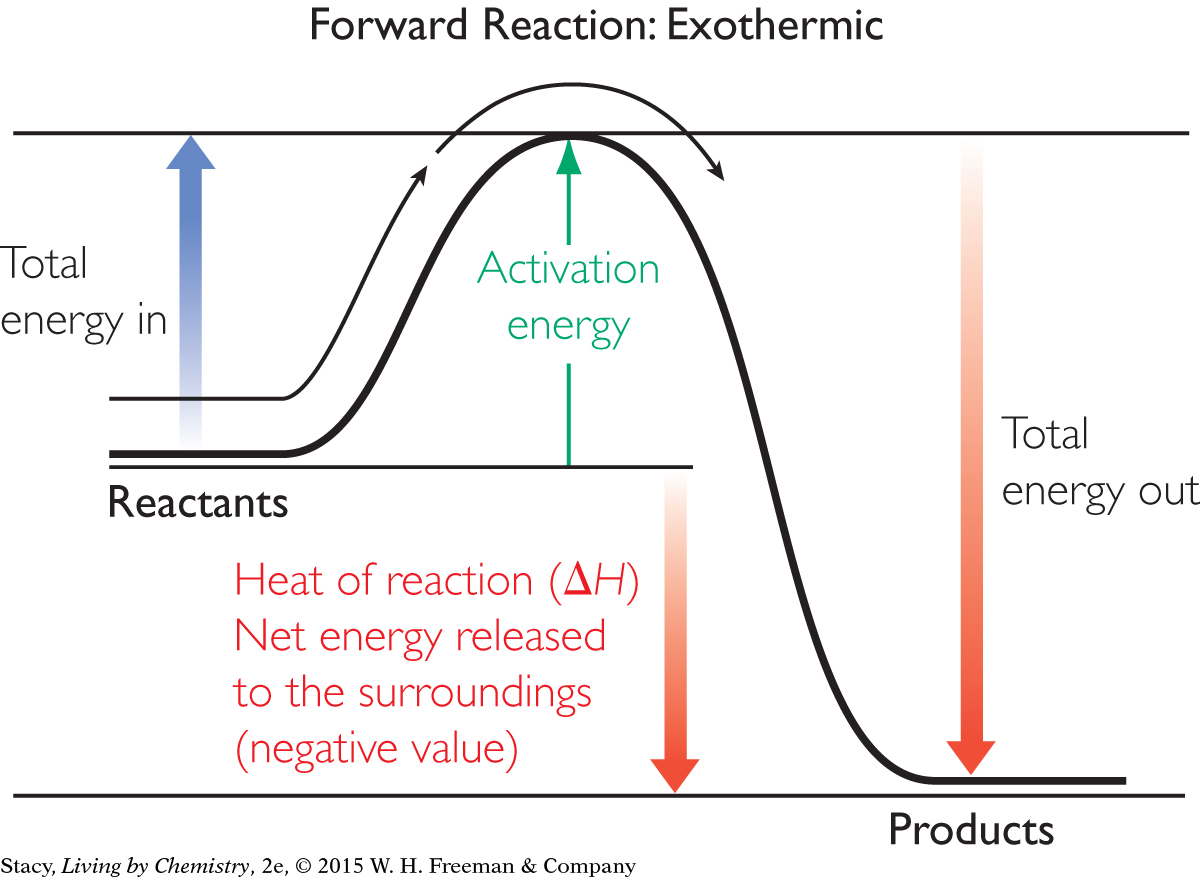
The potential energy between the reactants and products for this reaction is called the activation energy. Energy must be supplied to the reactants to get over this energy barrier. This is why reactions often require a spark to get started. Once an exothermic reaction is started, the reaction itself can provide the necessary energy for more molecules to react. This is why a single spark can cause an entire forest to burn down.
Big Idea
Big Idea
Some reactions are energetically favored over other reactions.
In contrast, energy must be supplied continuously to cause the reverse of an exothermic reaction. You can see from the diagram that the reverse of the exothermic reaction is an endothermic reaction. Notice also that the activation energy, or energy barrier, is much greater in the reverse direction.
538
The net energy change from reactants to products of this reaction does not provide the necessary energy to activate more molecules to react. This is why you need to continuously supply energy to decompose water to hydrogen and oxygen. Endothermic reactions generally are less likely to occur than exothermic reactions.
LESSON SUMMARY
LESSON SUMMARY
What is the energy associated with reversing reactions?
KEY TERMS
potential energy
activation energy
Energy is conserved in chemical processes. This means that the net energy exchange in a forward process is equal and opposite to the net energy exchange in the reverse process. If a forward reaction is exothermic, the reverse reaction is endothermic. Energy in a chemical system is in the form of either kinetic energy or potential energy. When substances burn, a great deal of potential energy is converted into kinetic energy. Reactions with lower activation energies are easier to get started.
Exercises
Reading Questions
What happens to the potential energy and the kinetic energy of a chemical system during an exothermic reaction?
Explain why you do not have to keep sparking a fire once it is started.
Reason and Apply
Consider the combustion of ethane, C2H6(g).
Write a balanced equation for the reaction.
Draw an energy diagram for the forward reaction. Label the reactants and the products. Use the table of heats of combustion from Lesson 103: Fuelish Choices to label the heat of reaction.
What is the net energy exchange of the reverse reaction? Explain.
Consider the energy diagrams below for the combustion of one mole of methane and the reverse reaction.
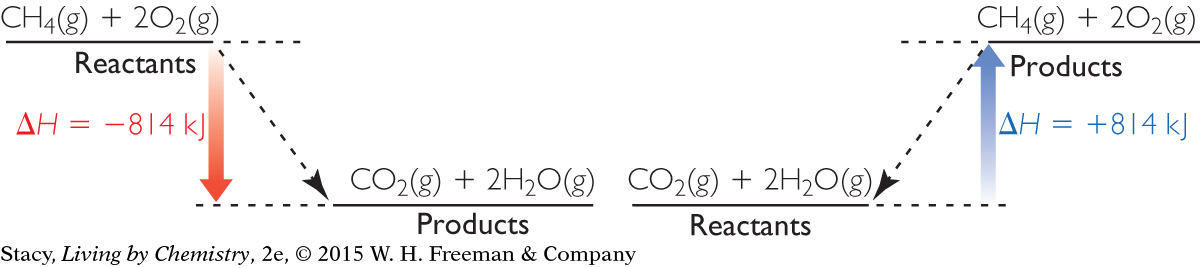
Which diagram represents an endothermic reaction?
Which substances have the lowest potential energy?
When methane reacts with oxygen to form carbon dioxide and water, the potential energy decreases. What happens to the kinetic energy?
Explain why the reverse reaction requires a constant input of energy.
Use the table of average bond energies from Lesson 104: Make It or Break It to estimate the heat of reaction. How does your value compare to the heat of reaction given in the diagram?
539
Copy the three energy diagrams shown here.
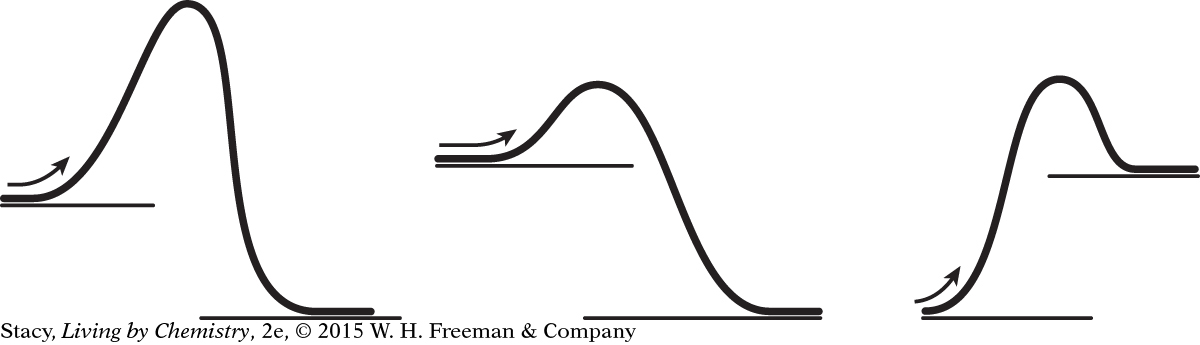
Label the heat of reaction on each.
Label the activation energy on each.
Which diagram represents the most exothermic reaction?
Which reactions require energy to get started?