LESSON 107: Make It Work
THINK ABOUT IT
544
All around us are machines doing work for us. They transport us from place to place, they help us to manufacture products, and they allow us to listen to music, wash our clothes, and watch television. The source of energy for most of these machines is chemical change.
How can a chemical reaction be used to do work?
To answer this question, you will explore
Work
Converting Chemical Energy into Work
Work
EXPLORING THE TOPIC
Work
To move anything, whether it is a truck or an atom, from one place to another, requires work. Work, like heat, is a transfer of energy. Specifically, work is the result of a force acting through a distance. A force is a push or a pull upon an object as a result of an interaction with another object. Force is measured in newtons, N. Scientists define mechanical work as the product of force and distance.
Work = force · distance
INDUSTRIAL CONNECTION
INDUSTRIAL
CONNECTION
The electricity that runs through the wires in buildings comes from a variety of sources, including hydroelectric, nuclear, and geothermal power plants. However, the majority of electricity used in the United States is generated from power plants that burn coal, oil, or natural gas to heat water and make steam. This pressurized steam turns giant turbines that generate electricity.

W = Fd
LIFTING AN OBJECT
Imagine that you pick up your baby sister, who weighs 22 pounds, or 10 kilograms, and lift her 1.2 meters. To lift her, you exert 100 newtons of force. This force is equal to the force of gravity acting on your sister, or her weight if it were expressed in newtons.
The work done picking up your 22-pound sister is equal to the force of her weight in newtons multiplied by the distance she was lifted.

Work = 100 N · 1.2 m = 120 N · m
Work is expressed in units of newton-meters, N · m. Work and energy are related. One newton-meter is equal to one joule.
545
Machines (or tools) help us to do work. Levers, ramps, pulleys, wheels, and wedges are all examples of simple machines. They give you a mechanical advantage to perform work. Consider how easy it is to lift a great deal of weight using a seesaw. A claw hammer acts as both a wedge and a lever, allowing you to pull a nail out of a board, something that would be nearly impossible with your bare hands.
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
Hydroelectric power plants, windmills, and solar panels do not use chemical reactions to produce electricity. These power sources depend on physical change alone. They are good alternatives to using fossil fuels to produce electricity.


ENERGY FROM CHEMICAL REACTIONS
Chemical reactions are sources of energy. Matter has the potential to transfer energy when it is transformed into new compounds. You have seen that burning different fuels can transfer large quantities of energy in the form of heat and light. But heat is not the only useful source of energy from chemical reactions. Consider the products of a combustion reaction. Both the gases formed and the heat produced are potential sources of work.
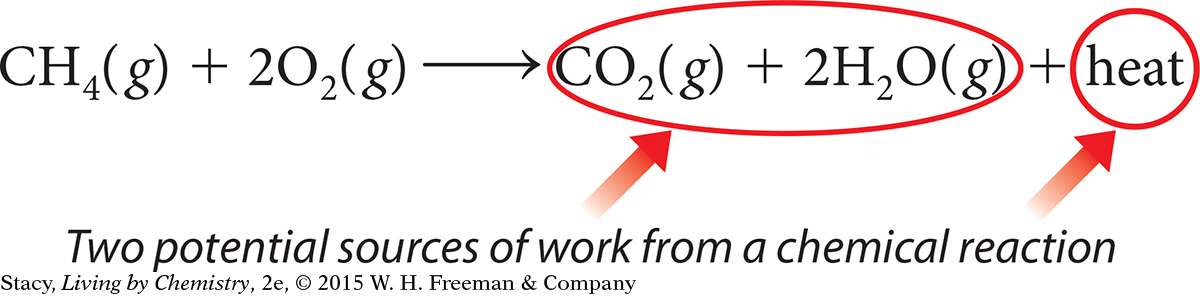
Recall from Unit 3: Weather that the pressure from expanding gases can be a powerful force. In the case of a combustion reaction, the products are in the form of heated gases that can expand and push on objects. The work done by expanding gases is often referred to as PV work, where P and V stand for pressure and volume. PV work is equal to pressure times the change in volume.
W = P Δ V
If pressure is expressed in atmospheres and the volume is expressed in liters, PV work is expressed in liter-atmospheres. Liter-atmospheres can also be converted to units of energy in joules.
1 liter-atmosphere = 101 joules
Converting Chemical Energy into Work
Converting Chemical Energy into Work
Important to Know
A system cannot contain or store either heat or work. So, heat and work both represent energy in transition.
Chemical systems are often paired up with machines to transform chemical change into useful work. The heat transferred, q, from a reaction or process can be utilized in some way, or the chemical products themselves can be used.
STEAM ENGINE
A steam engine uses a combustion reaction to heat water in a cylinder with a piston. When the heat is transferred to the water, the liquid changes phase to a gas that expands and moves the piston.
546
HISTORY CONNECTION
HISTORY
CONNECTION
The earliest recorded steam engine was built by an ancient Greek mathematician named Hero of Alexandria. The steam came out through two pipes, making a metal arm spin around. There is little evidence that this engine was used for anything more than amusement.

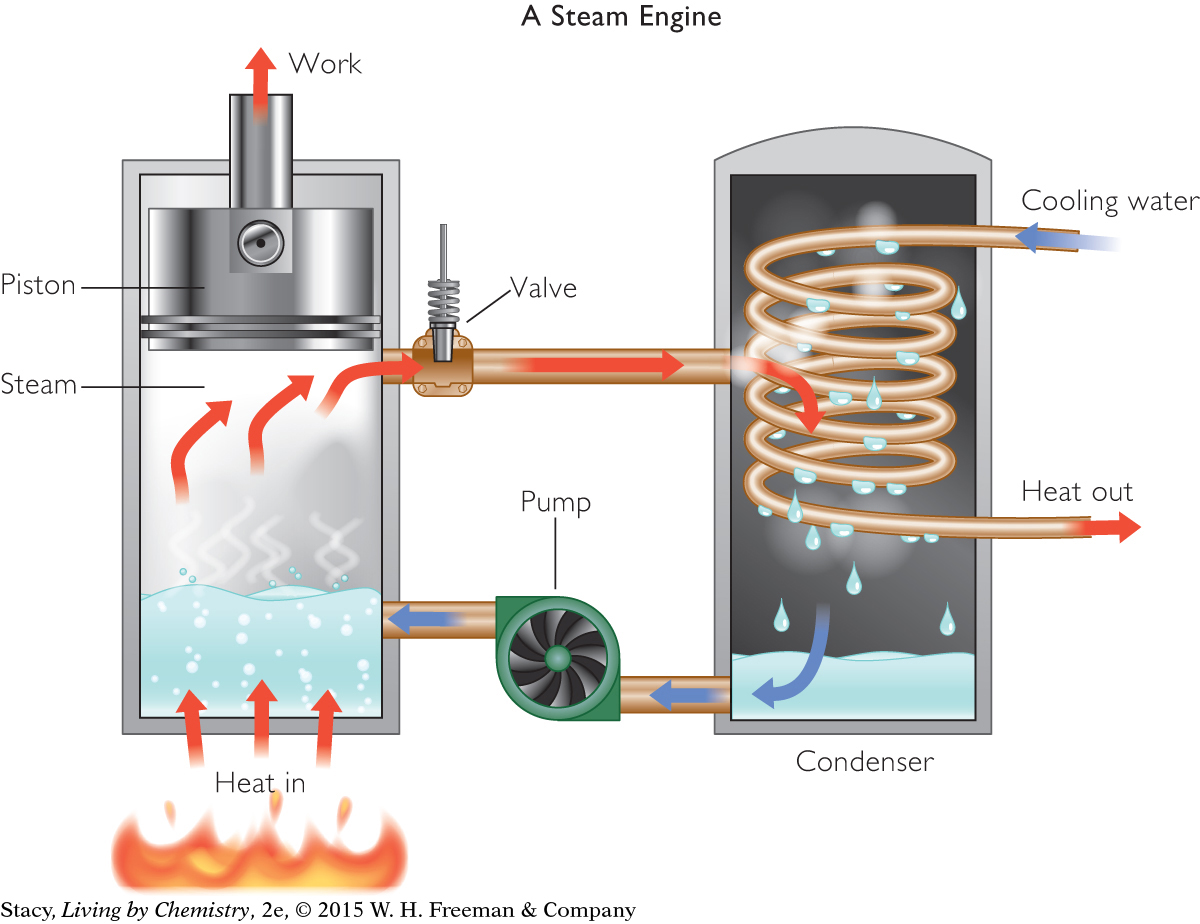
Notice that this is a two-step process as the heat from a chemical change is used to cause a physical change. The expanding gas (water vapor) does the work in this type of engine. Early steam engines were used to pump water and to power steamboats, trains, farm machinery, factories, and the first automobiles.
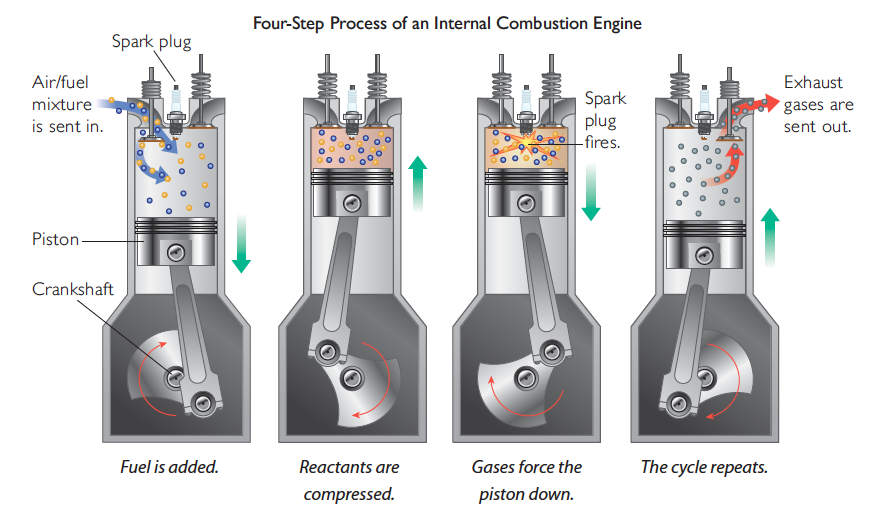
INTERNAL COMBUSTION ENGINE
Internal combustion engines like those in cars also make use of expanding gases. However, this type of engine uses the gases produced in the combustion reaction, carbon dioxide and water vapor, to do the work.
The illustration shows an internal combustion engine going through a four-step process.
547
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
The plant life on our planet converts solar energy to chemical energy through photosynthesis. Without photosynthesis, the petroleum products we use now as fuel would not exist.

This four-step process is repeated at an extremely rapid rate to run the engine. The expanding carbon dioxide and water vapor from the combustion reaction move the pistons up and down, turning a large crank that ultimately makes tires, wheels, propellers, or turbines go around.
ENTROPY
Internal combustion and steam engines convert thermal energy into work. As required by the first law of thermodynamics, the total energy is conserved in the process. However, it is impossible to completely convert all that energy into work. Some of the energy is lost to the surroundings as heat.
This loss of heat to the surroundings is related to entropy and the second law of thermodynamics. As you recall, the second law states that energy and matter tend to disperse or become more disordered. Entropy is the measure of the unavailability of a system’s energy to do work due to this dispersal. So, when designing an efficient machine, scientists and engineers must account for entropy.
Big Idea
Big Idea
Energy disperses; it does not collect.
LESSON SUMMARY
LESSON SUMMARY
How can a chemical reaction be used to do work?
KEY TERM
work
Work is force acting upon an object causing it to move in the direction of the force. So work is energy in motion. The heat transferred, q, from chemical reactions can be used to do work. The work done by expanding gases is called PV work. The combustion of fuels is one of the most common ways we transform chemical energy into work. However, some energy is always lost, due to entropy.
Exercises
Reading Questions
What is the scientific meaning of the word work?
Give an example of how a chemical reaction can be used to do work.
Reason and Apply
The expansion of gases pushes a piston. The volume of the gases increases from 0.5 L to 12.5 L. If the piston is pushing against a pressure of 1.2 atmospheres, how much work is done by the gases?
If it takes 45 N of force to lift a box 3.8 m, how much work is done to the box?
Research and describe three ways that a rocket engine is different from an internal combustion engine. What extra item must a rocket engine carry with it into space?