Chapter 20 Summary

CHAPTER 20
Understanding Energy
SUMMARY
KEY TERMS
bond energy
potential energy
activation energy
work
548
Fire Update
All chemical reactions involve the breaking of bonds and the making of bonds. The net energy difference between these two processes determines if a reaction is exothermic or endothermic. This is because bond breaking requires energy and bond making releases energy. By using average bond energies, you can closely approximate the heat of reaction, ΔH.
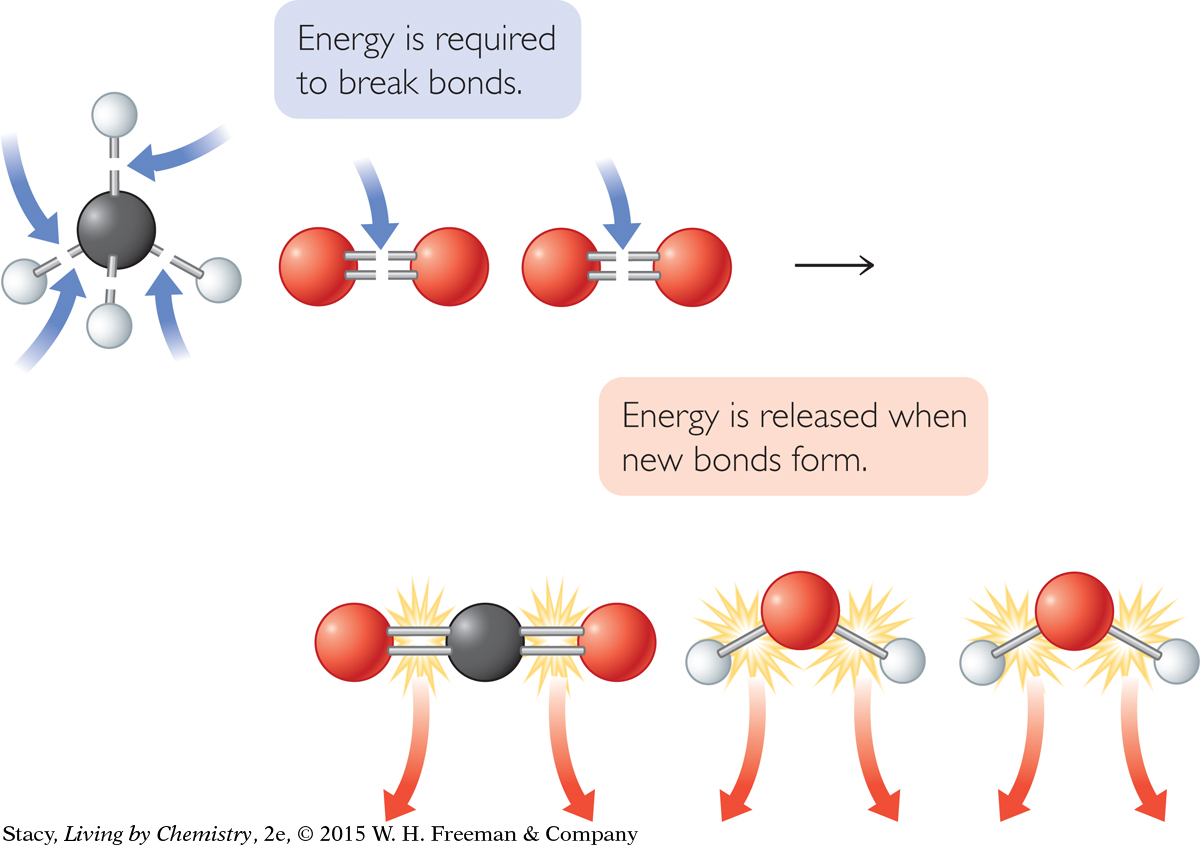
Each chemical system expresses energy in the motions of its molecules or particles. This is known as kinetic energy. In addition, each chemical system has potential, or the potential for further energy in rearranging its chemical bonds. When an exothermic reaction occurs, the system’s potential energy decreases and its kinetic energy increases. The reverse is true in an endothermic reaction. An increase in kinetic energy is accompanied by an increase in temperature.
It generally takes an input of energy, called the activation energy, to get a reaction started. This is why a fire requires a match or a spark. The overall conditions and the form that the reactants are in can affect the rate of a reaction. For example, raising the temperature, reducing the surfaces of reactants, or adding a catalyst can increase the rate of a reaction.
Chemical reactions can be used to do work. For example, the internal combustion engine uses the gaseous products formed in a combustion reaction to do work on pistons.
REVIEW EXERCISES
Question 20.1
1. List seven ways you could speed up a chemical reaction. Explain how each method can increase the rate of reaction.
Question 20.2
2. You have a cup of iced coffee that you are going to sweeten with a sugar cube. Name three ways that you could speed up the rate of dissolving sugar in the coffee. Explain why each of your methods works according to collision theory.
549
Question 20.3
3. You are walking by a classroom, and you overhear two students having a discussion about boiling water. Whom do you agree with and why?
Trey: When you’re boiling water, you’re breaking the bonds between the hydrogen and oxygen atoms in water. The steam you see above the boiling water is hydrogen and oxygen gas. Heating the water provides the energy to break these bonds.
Paige: Boiling water is not providing nearly enough energy to decompose water. The steam you see above the boiling water is still water. You have not broken any bonds to make it. Heating the water provides the energy to vaporize the water.
Question 20.4
4. The chemical formula for heptane is C7H16.
Draw the molecular structure for heptane.
Write the balanced equation for the combustion of heptane.
Using the table of average bond energies (located on page 531) and your answers to parts a and b, calculate the heat of reaction for heptane.
Question 20.5
5. Methane, CH4, is a combustible gas used in high school and college labs to burn in Bunsen burners. Carbon dioxide, CO2, is an incombustible gas used in fire extinguishers.
Draw the molecular structures for methane and carbon dioxide.
Using the table of average bond energies on page 531, calculate the amount of energy that would be needed to break the bonds in a mole of methane and a mole of carbon dioxide.
Why do you think that methane combusts, but carbon dioxide doesn’t?
Question 20.6
6. A balloon is placed over a flask with water in it. As the flask is heated on a hot plate, the balloon expands from a volume of 0.1 L to 1.6 L at a constant 1.1 atm.
Calculate the amount of work that the water molecules did on the balloon.
Is there anything else that needs to be taken into account when calculating the amount of work that the water vapor did?