Featured LAB: Electrochemical Cell
Featured LAB
568
Electrochemical Cell
!
SAFETY
Instructions
Wear safety goggles. Wear protective gloves for handling the salt bridge.
Purpose
To explore how to get electrical energy from a redox reaction.
Materials
zinc strip
copper strip
250 mL beakers (2)
500 mL beaker
2 connecting wires with alligator clips
tiny LED light bulb
1.0 M CuSO4, 100 mL
1.0 M zinc sulfate solution, ZnSO4, 100 mL
saturated potassium nitrate (KNO3) solution, 20 mL—for salt bridge
filter paper approximately 1 in wide and 6 in long—for salt bridge
gloves for handling salt bridge
Procedure
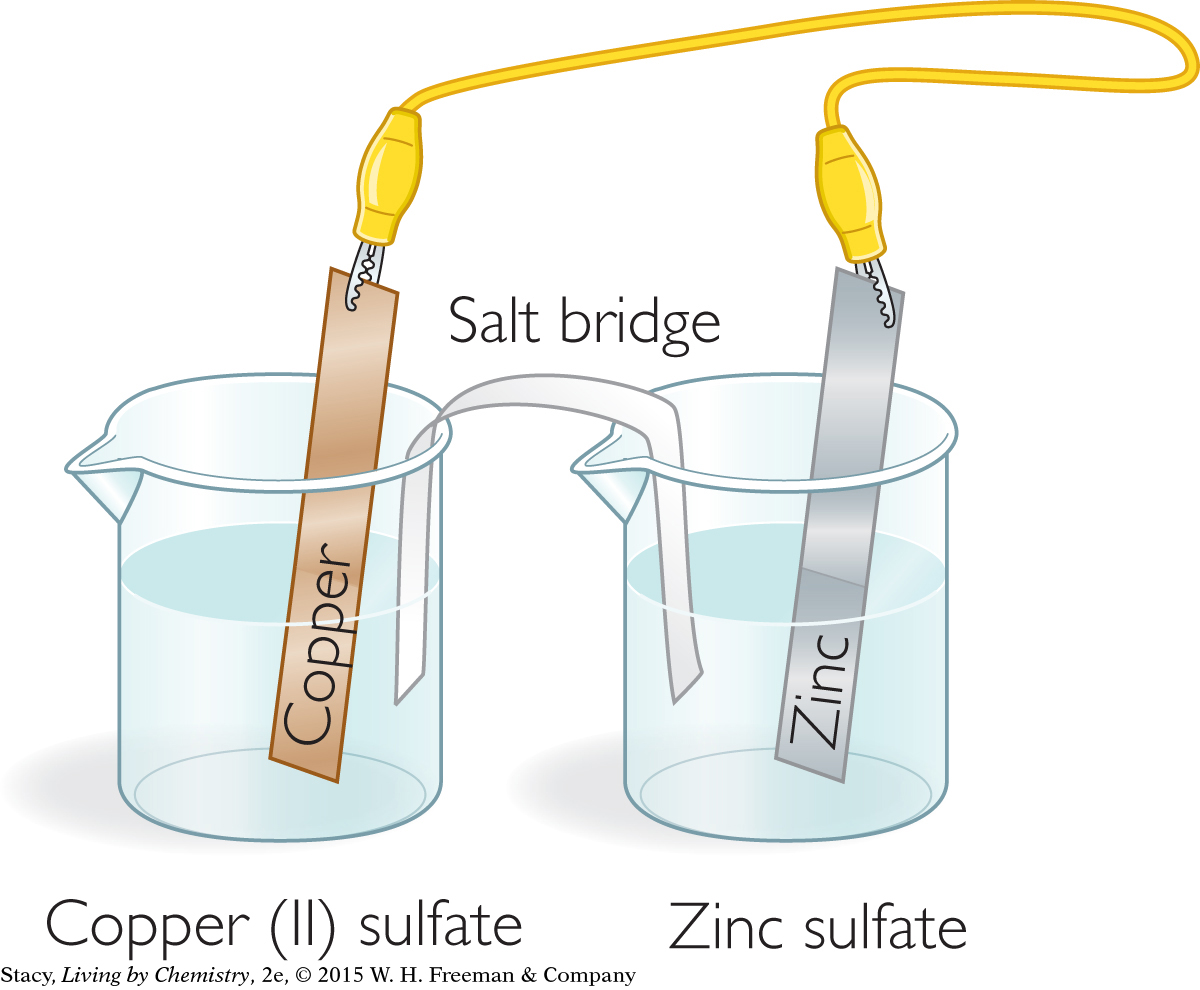
Make a salt bridge. Place a piece of folded-up filter paper in the bottom of an empty 500 mL beaker. Soak it thoroughly with KNO3. Set aside for later.
Carefully pour 100 mL of CuSO4 into one beaker and 100 mL of ZnSO4 into another beaker.
Set up the zinc strip in the ZnSO4 solution and the copper strip in the CuSO4 solution. Use a wire with alligator clips to connect the two metal strips. The clips should not touch the solutions.
Use gloves to place the salt bridge between the two beakers as shown.
Observation and Analysis
What do you observe?
Why is it necessary to have an ionic solution in the salt bridge?
Which substance is being oxidized? Which substance is being reduced? How do you know?
Connect the tiny LED light into your circuit using both sets of alligator clips. What do you observe? What does that prove?
Explain how you might reverse this reaction.
Making Sense Explain where the electricity in the electrochemical cell is coming from.