LESSON 115: Beyond What You See: Electromagnetic Radiation
586
THINK ABOUT IT
On a dark night, it is very difficult to find your way without bumping into objects that you cannot see. Put on a pair of night vision goggles and you can detect objects fairly easily. What do the goggles detect so that you can see at night?
What is the evidence for light waves that are invisible to your eyes?
To answer this question, you will explore
Electromagnetic Radiation
Light Energy

Science Photo Library
|

© David J. Green—technology/Alamy
|
Electromagnetic Radiation
EXPLORING THE TOPIC
Electromagnetic Radiation
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
Unlike human beings, bees can see ultraviolet light. Bee vision is shifted toward the blue range, and they do not see red. Flowers have adapted to guide bees toward pollen and nectar. The center of this creeping zinnia flower is an unmistakable target for a bee, which sees two-toned petals that humans do not see. As part of its reproduction process, the zinnia advertises that it has pollen for bees to collect for the hive and deposit in other flowers.

The human eye is able to detect a range of light waves with wavelengths from about 7.5 × 10–7 m for red light to 3.9 × 10–7 m for violet light. You might wonder if these are the only light waves that exist or if there are light waves with longer and shorter wavelengths outside your range of vision. The example of night vision goggles indicates that there is light even in the dark. However, human eyes cannot detect this type of light without the assistance of technology.
A LARGE COLLECTION OF WAVES
The light that we see is a small part of a large collection of waves called electromagnetic radiation. As indicated in the figure, electromagnetic radiation exists with a huge range of wavelengths and frequencies. Its frequency ranges from a low frequency of less than 105 Hz to a high frequency of greater than 1020 Hz. Its wavelength ranges from longer than 103 m to shorter than 10–12 m. These wavelengths have dimensions that are roughly equivalent to mountains for the longest wavelengths and to smaller than atoms for the shortest wavelengths.
587
All of these electromagnetic waves have one important thing in common: They all travel through space with the same speed, the speed of light, c = 3 × 108 m/s. In all cases, the frequency times the wavelength is equal to the speed of light.
f × λ = c = 3 × 108 m/s
Only waves that are classified as electromagnetic radiation travel at the speed of light.
Big Idea
Big Idea
Electromagnetic waves all travel with the same speed in a vacuum, the speed of light.
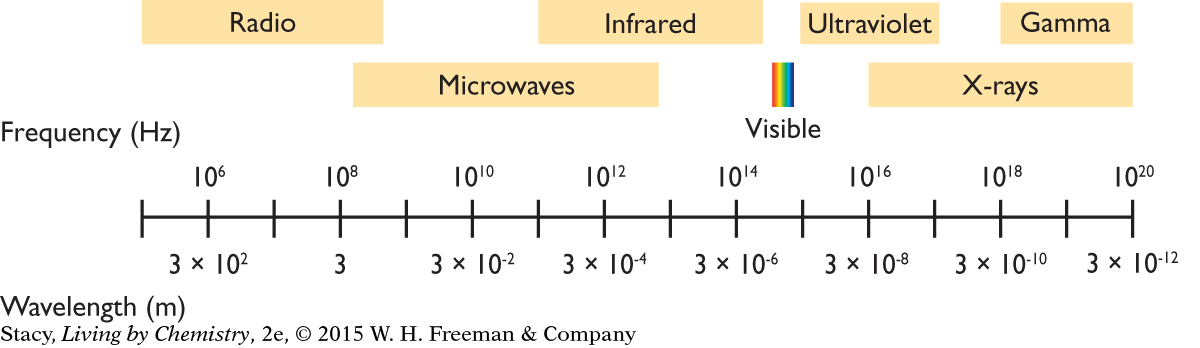
NAMES FOR ELECTROMAGNETIC RADIATION
The various wavelength ranges are classified with names, some of which are familiar because they have entered into everyday language. Here is a description of the names for the various wavelength ranges from the longest wavelengths to the shortest wavelengths. Notice that the words ray, wave, and radiation are used in the names.
Radio waves: Radio waves are emitted at a radio or television station. Radio waves are all around you all the time, but your body is unable to detect them. A device such as a radio or television converts radio waves into sound and picture. Wireless routers and wireless pointers also use radio waves.
Microwave radiation: Microwaves are emitted by the power source in a microwave oven. Water molecules absorb microwaves. This causes the water molecules to move. The motion raises the temperature of the water contained in food, which in turn causes the temperature of the food to increase.
Infrared radiation: Your body senses infrared radiation as heat. For example, if you stand in direct sunlight, you will feel hotter than if you stand in the shade. In both cases, the air temperature is the same. The difference is that in direct sunlight, you are exposed to infrared radiation in addition to visible light. Infrared, or IR, radiation causes molecules to vibrate. This vibration motion also causes the temperature to increase. You may be familiar with heat lamps, such as those used to keep pet reptiles warm. These lamps use IR radiation.
588
Visible light: Your eyes detect visible light as various colors. The colors of the rainbow are in the sequence of the shortest wavelength closest to Earth (violet) to the longest wavelength at the top of the arc (red).

Ultraviolet radiation: If you are out in the sun too long, your skin will turn red with sunburn. This does not happen with room lights, indicating that sunlight has additional waves with wavelengths outside the visible region. The waves that cause sunburn are called ultraviolet, or UV, radiation. Sunburn is damage to your skin. The UV rays cause molecules in your skin to break apart. Sunscreens provide protection by blocking the UV rays from getting to your skin.
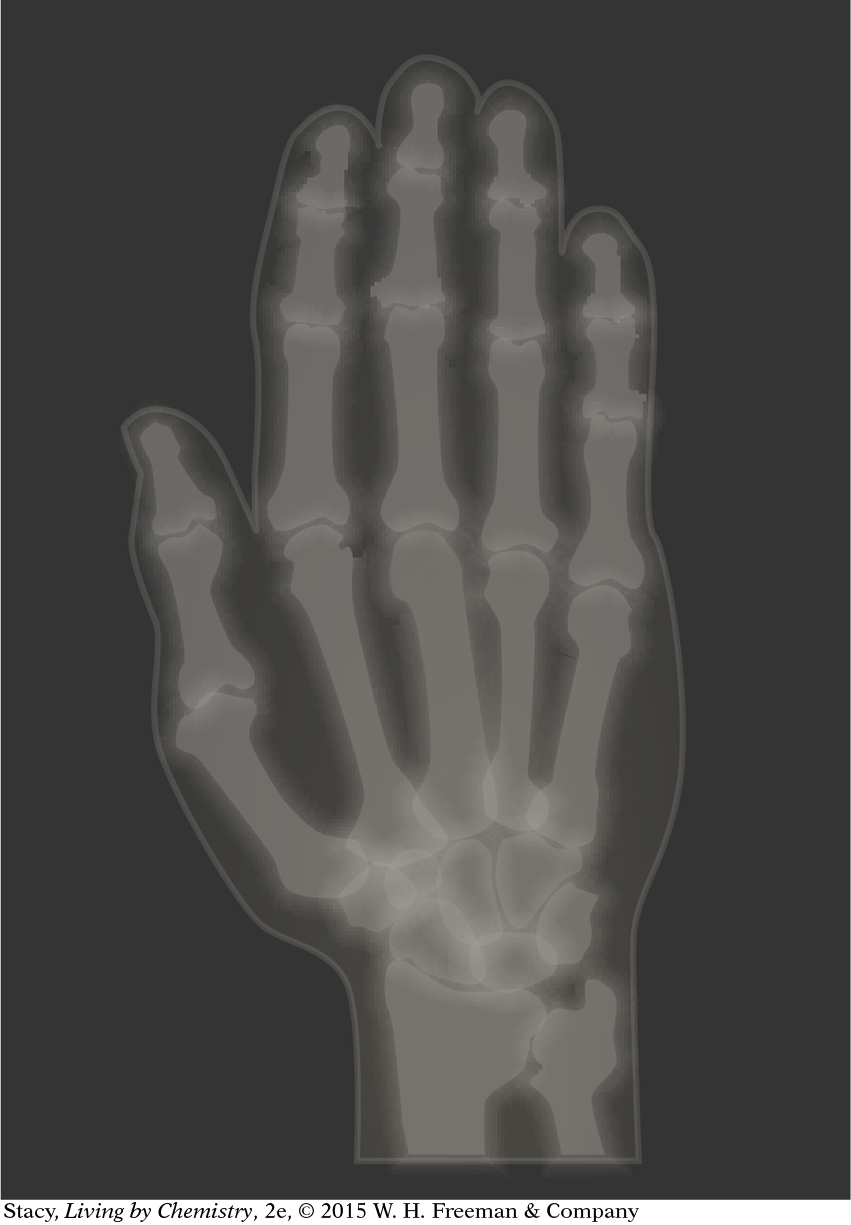
X-rays: If you have ever broken a bone, then you have probably had an x-ray photograph taken. X-rays are dangerous, so the x-ray technician keeps the exposure time short. The x-rays fly through most substances, but they are blocked by bones and teeth. By detecting the x-rays that come through your body, it is possible to capture a photograph of your bones to locate a break or a photo of your teeth to locate cavities. X-ray technicians and dentists place a lead apron on you to block x-rays from reaching parts of the body that are not under examination.

Gamma rays: Gamma rays have the highest frequencies and shortest wavelengths, and they are extremely dangerous. Gamma rays are emitted during a nuclear explosion, in which the nuclei of atoms are falling apart.
Light Energy
Light Energy

Consider a fire caused by a chemical reaction between wood and oxygen to produce carbon dioxide and water. The reaction is exothermic, and it transfers a lot of heat to the surroundings. How does this transfer of heat occur?
So far in this unit, you have considered energy transferred by direct contact between two objects at different temperatures. Energy is transferred by conduction until the two objects are at the same temperature.
Energy is also transferred when light interacts with matter. This is referred to as transfer of energy by radiation. IR radiation is especially efficient at transferring energy to make you feel warm. Fire emits a lot of IR radiation in addition to emitting visible light. Again, while you cannot see IR radiation, your body does detect it as heat.
Big Idea
Big Idea
Electromagnetic radiation is one way that energy is transferred from a source to an object.
RELATIONSHIP BETWEEN FREQUENCY AND ENERGY
The table shows examples of values for the wavelength, frequency, and energy of various types of electromagnetic radiation. The energy is measured in joules, J. One joule is roughly equivalent to 0.24 calories. This is enough energy to raise the temperature of 1 mL of water by 0.24 degrees.
589
Take a moment to notice the relationships among wavelength, frequency, and energy.
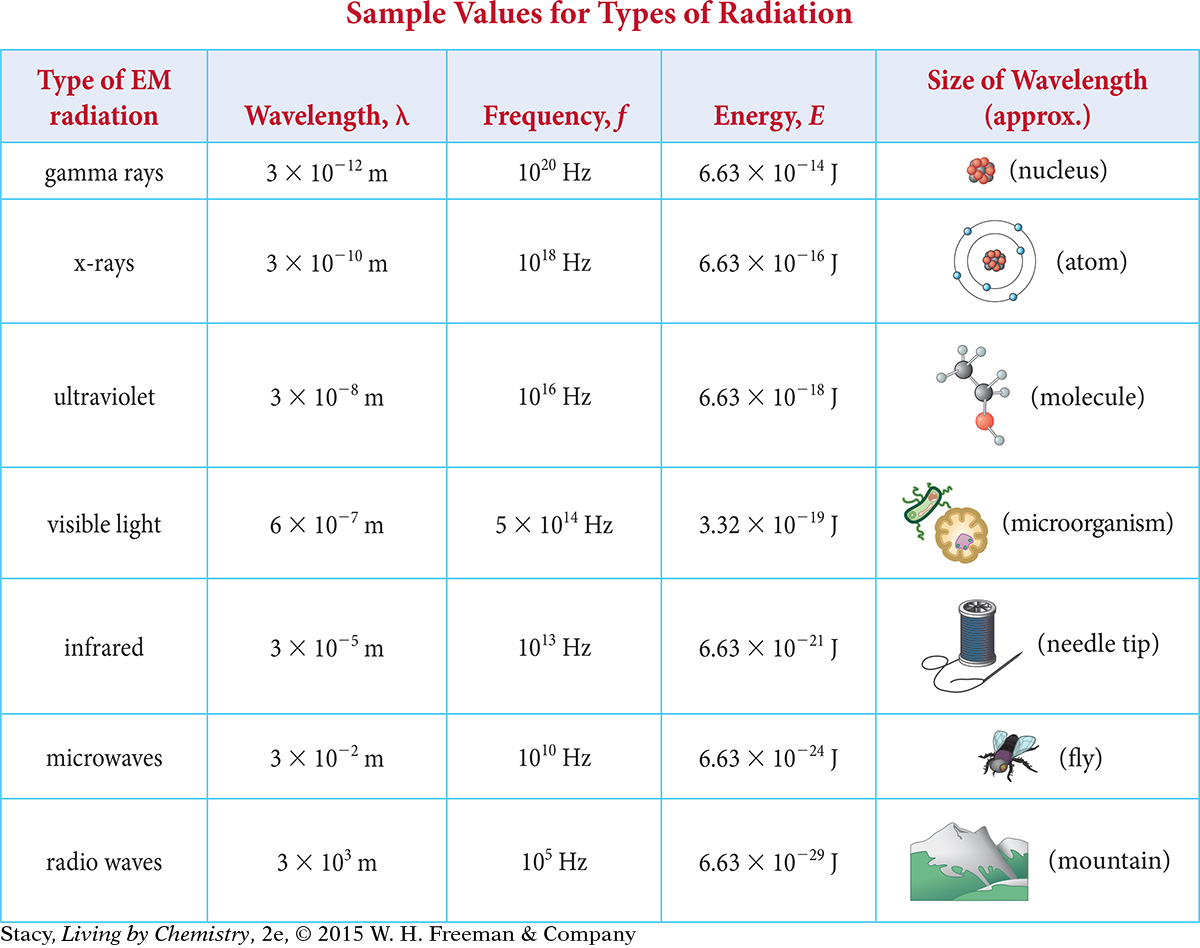
The energy of light increases as the frequency increases and as the wavelength decreases. One way to remember this is by considering that to make a high-frequency wave with the rope, you made a rapid back-and-forth hand motion. It definitely required a greater input of energy to generate a higher frequency.
PLANCK’S CONSTANT
Planck’s constant relates the frequency of the electromagnetic wave with its energy. It is given the symbol, h, defined as h = 6.63 × 10–34 J · s. The energy of the electromagnetic wave is calculated with the formula given below. For a frequency in hertz (s–1), the energy transferred by each wave (one up-and-down motion), is given in joules.
E = h × f
Frequency and wavelength are related by the equation c = f × λ. Rearranging the equation gives
f = c/λ.
Substituting c/λ for the frequency in the equation E = h × f gives the relationship between energy and wavelength.
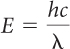
590
While each wave (one up-and-down motion) only transfers a tiny amount of energy, keep in mind that the waves are coming at a high frequency and with a high speed. So, the total energy transferred can be quite large.
PHOTON MODEL OF LIGHT
So far, you have considered a ray model and a wave model to describe light. A photon model is a third model to describe light. It is useful to think about one up-and-down motion of the wave as a “particle,” or photon of light. Instead of imagining light as a continuous light ray, the photon model suggests that a beam of light is composed of small particles.
According to the photon model, photons associated with waves of higher frequency transfer a greater amount of energy per photon. An x-ray photon transfers more energy than an infrared photon because the x-ray has a higher frequency compared with IR radiation.
Further, the photon model helps to explain the brightness of a beam of light. If the light is brighter, there should be a greater number of photons in a given area in one second. Light that is dimmer has fewer photons in the same area in one second.
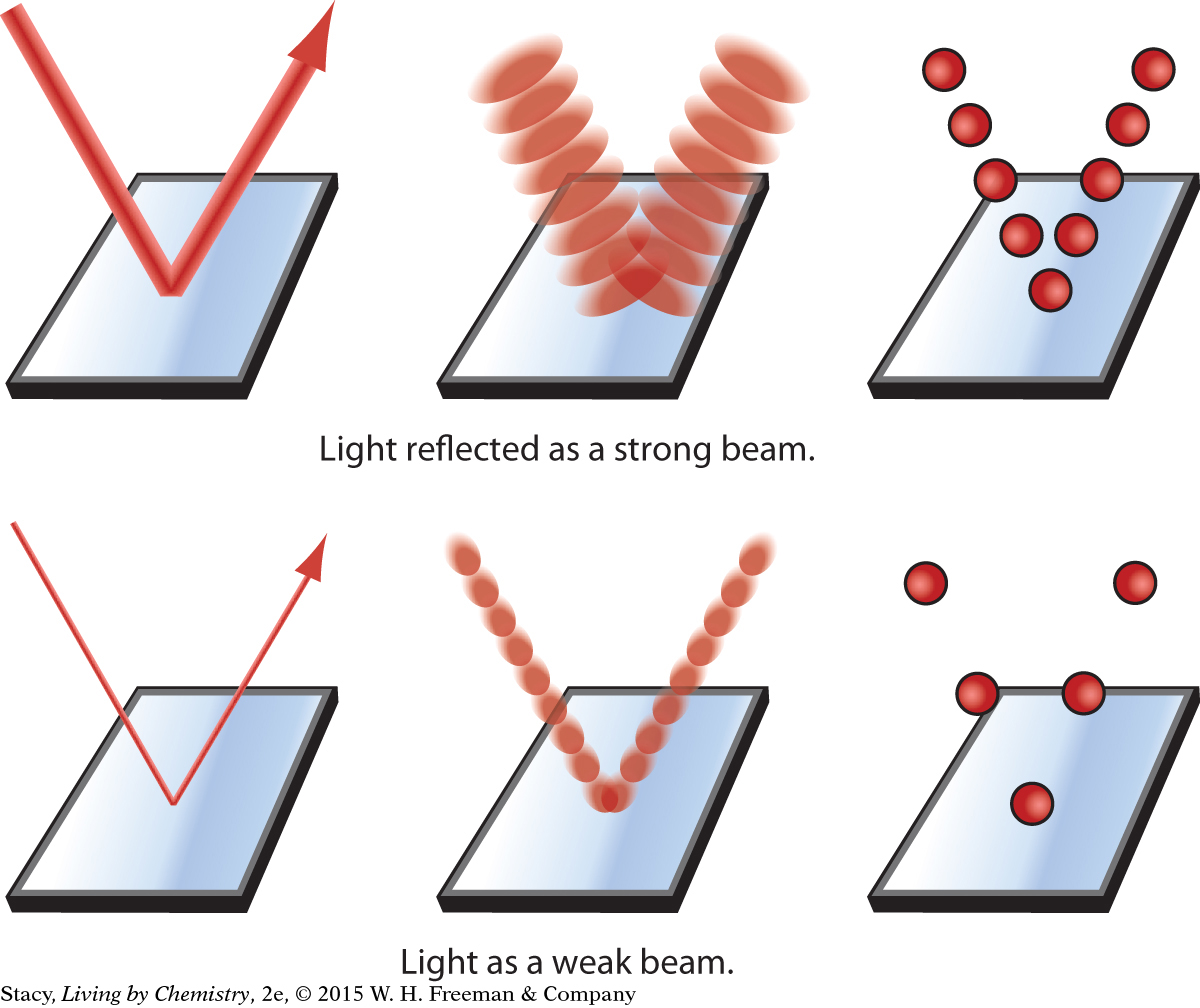
There are two ways of transferring more total energy in a given amount of time: (1) make the light brighter so that there are more photons, or (2) choose light of a higher frequency such that each photon transfers more energy. For example, bright red light has a greater number of photons compared with dim red light. Each photon transfers the same amount of energy, but there are more photons in the bright light. In comparison, even dim x-rays transfer a lot of energy because each photon transfers a much larger amount of energy than a photon of red light. The frequency of an x-ray is 1018 Hz, whereas the frequency of red light is only 1014 Hz. Each x-ray photon transfers about 10,000 times more energy compared with a photon of red light. This is why x-rays are dangerous.
Important to Know
Wave-particle duality simply indicates that both a wave model and a particle model describe electromagnetic radiation.
591
The wave model and the photon model are both useful. Scientists refer to the wave-particle duality of light, but this simply means that you can choose either model. Observations of radio waves indicate that they behave most like waves. In contrast, observations of the behavior of x-rays indicate that they are more particle-like.
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
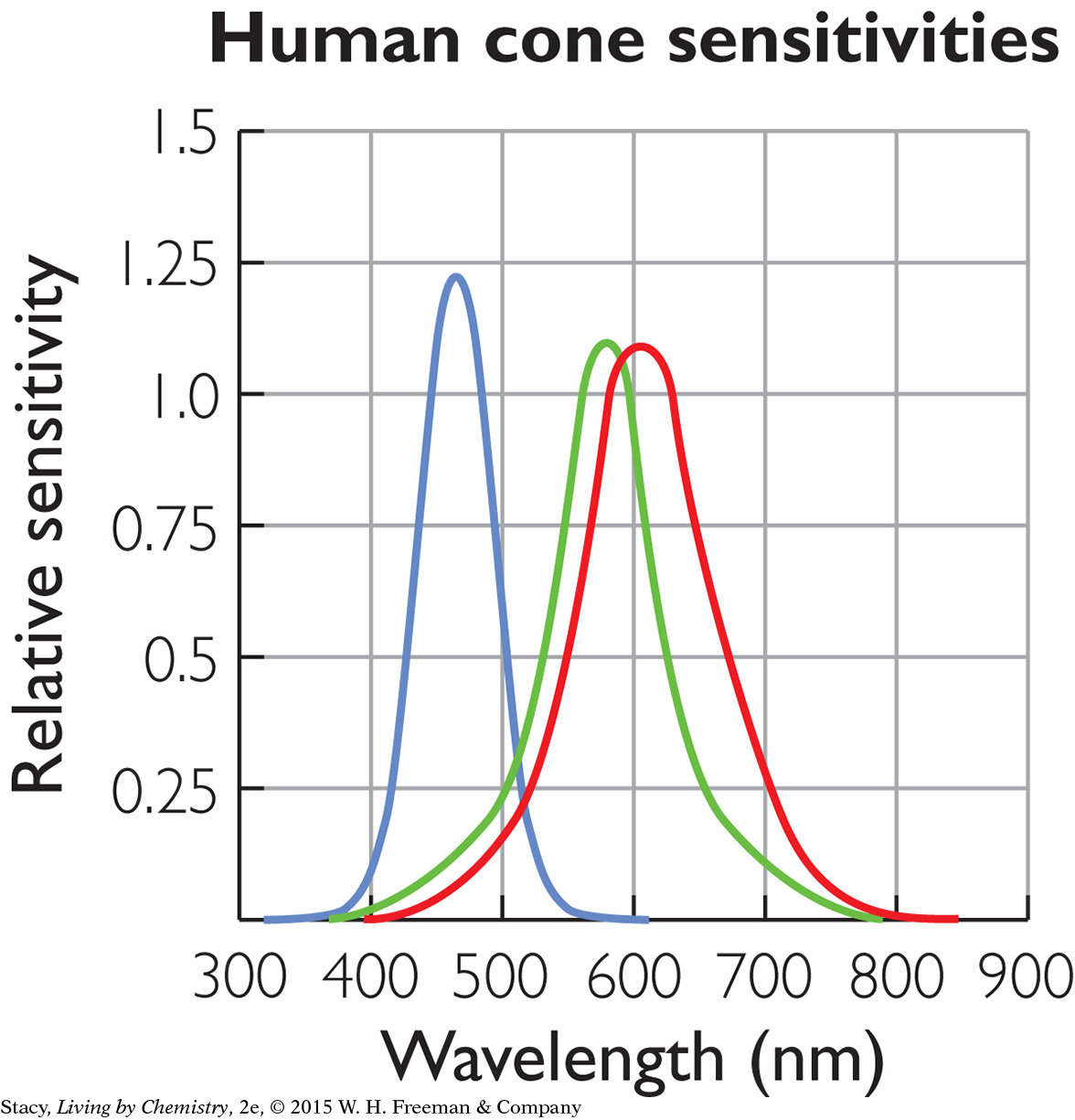
The human eye detects colored light by using three receptors that have maximum frequencies for red, green, and blue. When light strikes these receptors at the back of the eye, a signal is sent along nerves to the brain. The brain then takes the information from the three receptors to create the perception of various colors. (And unlike bees, human beings can see red.)
Example
Energy of Colored Light
Imagine a light wave with the frequency of 1017 Hz.
What is the energy of this electromagnetic wave?
Use the figure on page 587 and the table on page 589 to determine the type of electromagnetic radiation.
Solution
The energy and frequency of electromagnetic radiation are related by the equation:
E = h × f, where h = 6.63 × 10–34 J ·s
Substitute values for the Planck’s constant, h, and the frequency of light:
Energy = E = (6.63 × 10–34 J · s)(1017 Hz) = 6.63 × 10–17 J
Notice that seconds cancel because Hz = 1/s, giving units of joules.
The energy and frequency indicate that the electromagnetic radiation is x-rays.
592
LESSON SUMMARY
LESSON SUMMARY
What is the evidence for light waves that are invisible to your eyes?
KEY TERMS
electromagnetic radiation
radio wave
microwave radiation
infrared radiation
visible light
ultraviolet radiation
x-ray
gamma ray
Planck’s constant
photon model
The light that we see is a tiny part of a large collection of waves called electromagnetic radiation. Electromagnetic radiation exists with a huge range of wavelengths and frequencies. The wavelength regions include radio waves, microwave radiation, infrared radiation, visible light, ultraviolet radiation, x-rays, and gamma rays. The entire collection of waves travels at the same speed, the speed of light, c = 3 × 108 m/s. The energy transferred by electromagnetic radiation is proportional to the frequency of the wave. Electromagnetic waves of high frequency transfer more energy than waves of low frequency. High-frequency electromagnetic waves such as gamma rays and x-rays are quite dangerous because of the large amount of energy they transfer, even with a dim beam.
Exercises
Reading Questions
Summarize what you learned about electromagnetic radiation.
Describe the ray model, the wave model, and the photon model of light.
Describe the relationship between energy and frequency.
Reason and Apply
What type of radiation has a wavelength of 6.12 × 102 m?
Identify an object that is similar in size to the wavelength of a microwave.
A radio station broadcasts at a frequency of 790 MHz (7.9 × 108 s–1). What is the wavelength of the electromagnetic radiation generated by this radio station?
The gamma rays emitted by 60Co are used in radiation treatment for cancer. The frequency of these gamma rays is 3 × 1020 s1. What is the energy of this radiation?