LESSON 117: How Awesome
THINK ABOUT IT
609

The same “red” cabbage plant is known by different colors in various regions, depending on the pH of the soil in which it is grown and how people describe the color in their native language. The leaves can be reddish, purple, or blue. If you cook red cabbage, it normally turns blue. But you can get the red color back by adding vinegar to the pot. What is the chemistry behind these color changes?
What do color changes indicate?
To answer this question, you will explore
Molecular Views of Indicator Color Changes
Chemical Symbols to Represent Indicator Color Changes

Elzbieta Sekowska/Shutterstock
|

Viktor1/Shutterstock
|
Molecular Views of Indicator Color Changes
EXPLORING THE TOPIC
Molecular Views of Indicator Color Changes
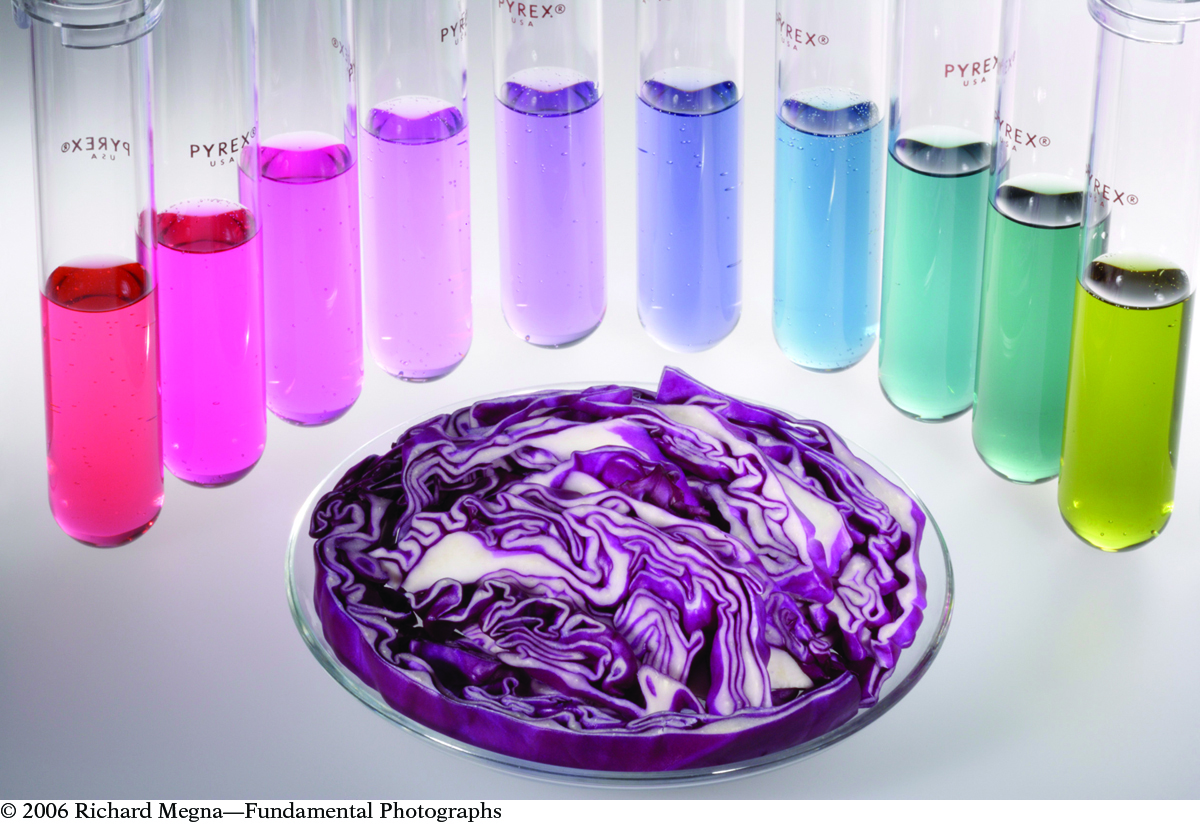
In Unit 4: Toxins, you explored cabbage juice as an indicator of the pH of a solution. As shown in the figure, the color of the cabbage juice indicator changes from red at pH ~3, to purple at pH ~6, and to blue at pH ~9. At higher pH values, the yellow color of a second indicator molecule is evident.
THE PIGMENT ANTHOCYANIN

A pigment is a substance that is colored because of the light it reflects. Instead of reflecting all the colors in white light from the light source, a pigment only reflects certain colors. For example, the leaves of red cabbage contain a pigment that reflects red light. The pigments in red cabbage are called anthocyanins. Anthocyanins are found in blueberries, cranberries, raspberries, cherries, grapes, eggplant, and the flower petals of violets.
610
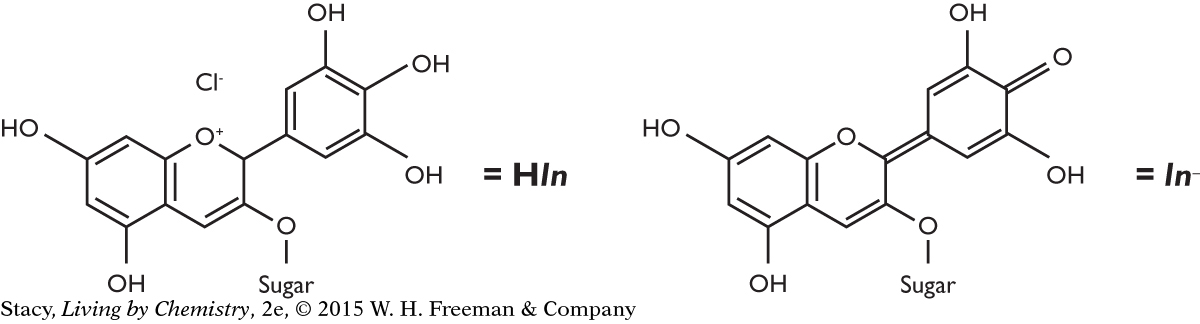
The anthocyanins that give red cabbage its color are large, complex molecules. Instead of writing out their molecular formulas, it is common to abbreviate the anthocyanin molecule as HIn. HIn is a weak acid, which means that it partially dissociates (breaks apart) into H+ cations and In− anions when dissolved in water. The HIn anthocyanin molecule is a red color. The In− anions that form when the anthocyanin molecules dissociate are a blue color.
Anthocyanins are called indicators because they change color when the pH of the solution they are dissolved in changes. For simplicity, all indicators in this unit will be abbreviated as HIn. And for the purpose of this lesson, we will refer to the HIn molecule as one form of the indicator, and the In− ion as a second form. The color of a solution that the indicator is added to depends on how much of each form of the indicator is present in that solution.
CHANGES IN COLOR
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
Besides its use as an indicator, anthocyanin is responsible for spectacular fall foliage. At the end of the growing season, when the chlorophyll in plants and trees decays, the red and purple colors of anthocyanins become visible, contributing to colorful foliage displays. Every autumn, “leaf peeping” draws many people to Vermont.

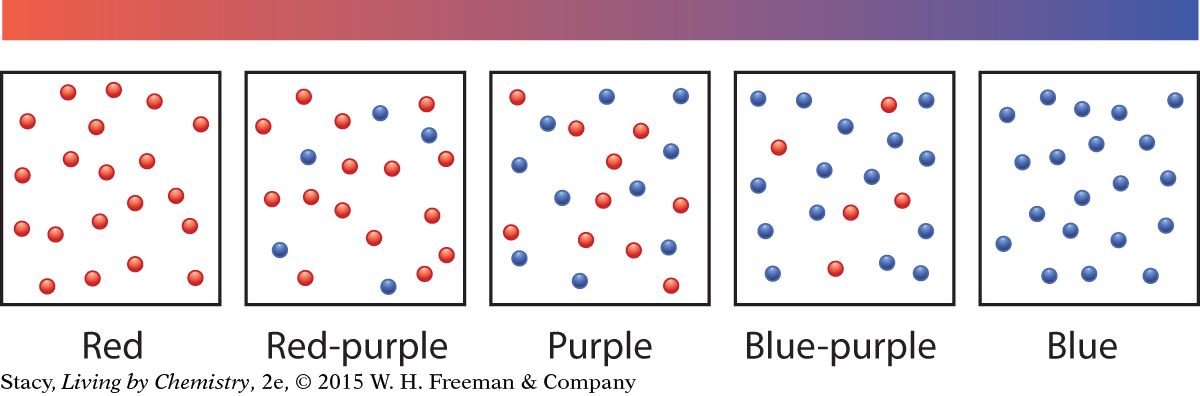
When an acid-base indicator is placed in an acidic solution, mostly HIn molecules will be present in the solution. When the indicator is placed in a basic solution, mostly In− anions will be present in the solution. The anthocyanin indicator is red in acid (HIn) and blue in base (In−). When the indicator is placed in a neutral solution, both molecules and anions, HIn and In−, are present. Because both the red and blue forms are present, the mixture appears purple. If the indicator is placed in a solution that is slightly acidic, the solution contains slightly more red HIn molecules, and its color is red-purple. If it is placed in a solution that is slightly basic, there are more blue In− anions, and its color is blue-purple.
Molecular views of a tiny volume of solution are shown in the figure on the next page. There are 20 anthocyanin molecules in this tiny volume, either as HIn molecules (red circles) or as In− anions (blue circles). Notice that there are no purple circles. Solutions that are varying shades of purple do not contain molecules that reflect purple light. Instead, these solutions reflect red and blue light. Our brains perceive the combination of red and blue as purple.
Big Idea
Big Idea
The color of an indicator solution varies with pH because the solution contains varying mixtures of HIn molecules and In− anions.
611
Chemical Symbols to Represent Indicator Color Changes
Chemical Symbols to Represent Indicator Color Changes
Molecular views are a good way to illustrate how the mixture of molecules in solution relates to the observation of solution color. Chemical equations are also useful for expressing the color changes that occur with indicator molecules in solution.
ANTHOCYANIN IN A BASIC SOLUTION
When anthocyanin is added to a solution that is basic, with pH ~9, the solution turns blue because the indicator is present mostly as In− anions. If H+ ions are added to the basic solution with In− ions, H+ combines with In− anions to form HIn molecules. The HIn molecule is red. As blue In− anions are converted to red HIn molecules, the solution appears varying shades of purple. Once all the In− anions have combined with H+, the solution turns red. The following balanced chemical equation describes the process that occurs as H+ ions are added.
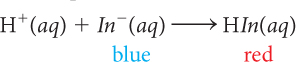
ANTHOCYANIN IN AN ACIDIC SOLUTION
When anthocyanin is added to a solution that is acidic, the solution turns red because the indicator is present mostly as HIn molecules. If OH− anions are then added to the acidic solution, the solution changes color from red to purple to blue. This is evidence that the number of In− anions in the solution increases as the pH increases. A reasonable conclusion to draw from these observations is that adding base to an acidic solution reverses the process described above of adding an acid to a basic solution. The following balanced chemical equation describes the process that occurs as OH− is added.
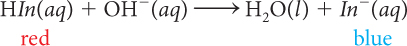
Although this equation does not appear to be an exact reversal of the first equation that describes adding H+, it can be written as two equations: one that shows that OH− combines with H+ to make H2O, and another that shows the HIn molecules dissociate into H+ and In− ions.
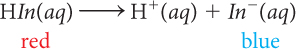
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
In a few species of plants, the color of the flower depends on chemistry. The flowers of hydrangeas, especially the species Hydrangea macrophylla, tend to be blue in acidic soil (pH below about 6) and pink in neutral and alkaline soil (pH above about 7). What does the color of these blossoms indicate?

H+(aq) + OH−(aq) → H2O(l)
612
These two chemical equations describe the same process as the single chemical equation above for adding OH−. It is possible to show this by adding the two chemical equations and canceling identical substances on both sides of the arrow.

It is easy to see now that adding acid to a basic solution of anthocyanin and adding base to an acidic solution of anthocyanin are processes that are the reverse of one another.
| Anthocyanin in a Basic Solution | Anthocyanin in an Acidic Solution |
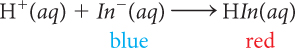
|
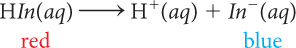
|
LESSON SUMMARY
LESSON SUMMARY
What do color changes indicate?
KEY TERMS
indicator
pigment
Color changes can provide information about chemical processes on a molecular level. One class of color-changing molecules is called an indicator. An indicator is a large, complex molecule that is abbreviated with the symbol HIn. When placed in solution, indicator molecules can exist as HIn molecules or as In− anions. This is because the HIn molecules can dissociate into H+ and In− ions. The reverse process can also occur in which the H+ and In− ions combine to form HIn molecules. In an acidic solution, there is plenty of H+ such that the indicator is present as HIn molecules. Adding base removes H+ to produce In− anions. Because the HIn molecules and In− anions are different colors, the color of the solution indicates the acid-base properties of the solution.
Exercises
Reading Questions
Explain how molecules account for the purple cabbage leaves.
Explain how the two forms of an indicator are related to one another.
Reason and Apply
Methyl red is an indicator that is red in acidic solution and yellow in basic solution. Draw three molecular views to show how the color changes from red to yellow. What color do you observe in between?
Write a balanced chemical equation to describe what happens as the color changes from red to yellow for methyl red indicator using the general symbols for an indicator molecule and its anion.
Write a balanced chemical equation to describe what happens as the color changes from yellow to red for methyl red indicator.
Research an acid-base indicator and explain how it works. (Do not use the universal indicators bromothymol blue, methyl red, or anthocyanin.)