LESSON 120: How Favorable
626
HEALTH CONNECTION
HEALTH
CONNECTION
Sneezing? When the body releases histamine into the nasal passages, the well-known “runny” nose occurs. Histamine is important as the immune system fights allergens. So histamine isn’t just a nuisance. It is a neurotransmitter involved in regulating many bodily responses. We can take antihistamine medicine to block the binding of histamines to shut down the “runny” nose response to the flood of histamines released.

THINK ABOUT IT
In a reversible chemical process at equilibrium, the starting substances and the products are both present as a mixture. In some processes at equilibrium, there are more products than starting substances present in the mixture. In other processes, there are more starting substances than products in the equilibrium mixture. Is there some way to predict whether more starting substances or products will be present when a chemical process reaches equilibrium?
How can you predict if products are favored in a reversible reaction?
To answer this question, you will explore
Products versus Starting Substances
Equilibrium Constant K
Dissociation of Strong and Weak Acids into Ions
Products versus Starting Substances
EXPLORING THE TOPIC
Products versus Starting Substances
Certain pain relievers act by attaching reversibly to receptors to block molecules that cause the pain signals from being sent to the brain. The strength of the binding between the pain reliever and its receptor site influences how effective the pain reliever will be. The stronger the binding, the longer the pain reliever stays in the receptor site, thereby blocking the molecules that cause the pain signal.
Consider the particle views of the binding of three different pain relievers. What do you think the equilibrium constant K indicates about the pain reliever?
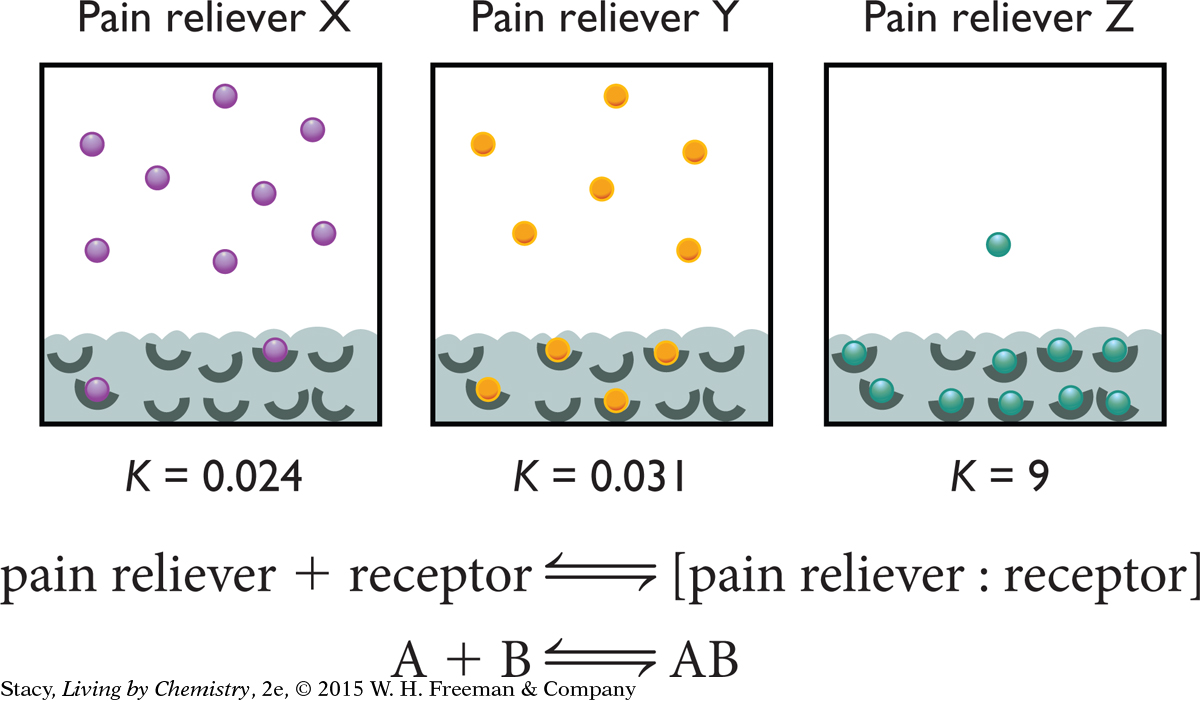
627
The figure shows that the equilibrium constant K is smallest for pain reliever X and greatest for pain reliever Z. For pain reliever X, 8 out of 10 pain reliever molecules are unbound. For pain reliever Z, only 1 molecule out of 10 pain reliever molecules is unbound. Although other factors might be important, it is likely that pain reliever Z is more effective at relieving pain per mole because more molecules are bound to receptors. It is reasonable to conclude that a small value of K suggests that fewer pain reliever molecules are bound, and therefore, the pain reliever is less effective.
Equilibrium Constant K
Equilibrium Constant K
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
For many years, scientists thought of sleep as something of a mystery. One clue for understanding sleep is the neurotransmitter melatonin and its sensitivity to levels of light perceived by the eye. In the bodies of mammals, melatonin levels vary over the course of the day, declining at twilight to produce drowsiness. Humans’ sleep-wake cycle is governed by changes in chemical equilibrium as melatonin molecules attach to and detach from receptors on the nerve cells to regulate sleep.

As you have learned, many processes in the human body involve the attachment of molecules to receptors. For example, molecules bind reversibly to receptors to regulate sleep, memory, mood, muscle contraction, and pain. In studying these processes in the human body, the concepts of equilibrium and reversible processes are relevant.
The equilibrium constant K is a measure of whether starting substances or products are favored in an equilibrium mixture. A large value of K indicates a large amount of product in the equilibrium mixture. A small value of K indicates very little product in the equilibrium mixture. A value of K can be determined experimentally for each reversible process. Because K describes the concentrations of starting substances and products in the equilibrium mixture, it is a valuable concept in making predictions about equilibrium mixtures.
Big Idea
Big Idea
The equilibrium constant K is a measure of whether starting substances or products are favored in an equilibrium mixture.
Example 1
Pain Relievers and K
Explain how the value of K relates to the particle views for pain relievers X, Y, and Z shown on page 626.
Solution
The smaller the value of K is, the more the starting substance is favored in the equilibrium mixture. In this case, the starting substances are individual molecules and empty receptor sites. For the larger value of K, the products are favored in the equilibrium mixture. In this case, the product is the pain reliever attached to the receptor.
Dissociation of Strong and Weak Acids into Ions
Dissociation of Strong and Weak Acids into Ions
The molecules in substances called acids both dissolve in water and dissociate into H+ and an anion. The word dissolve indicates that water molecules surround the acid molecules, and the acid molecules spread out in the solution. While salts dissolve and dissociate into ions, most molecular substances dissolve, without dissociating, like sugar. Nothing further happens.
628
Acids are special because even though they are molecular substances, they dissociate into cations and anions when they dissolve. This is shown in the particle views for tiny volumes of hydrochloric acid, HCl, solution. The solution is mostly H+ and Cl− ions, with no measurable number of HCl molecules.
Because acid molecules dissolve in an aqueous solution, and they also dissociate, you might wonder what solution molarity refers to in these cases. For example, the particle view of 0.010 M HCl does not have any molecules of HCl. Instead, the solution consists of 0.010 M H+ and 0.010 M Cl− because all of the molecules are dissociated into ions.
Important to Know
Hydrochloric acid is referred to as a strong acid, which means a strongly dissociated acid. Formic acid is called a weak acid, which means a weakly dissociated acid.
Formic acid, HCOOH, also dissolves and dissociates. However, as shown in the particle view, the solution is a mixture of HCOOH molecules, H+ ions, and HCOO− ions. This is because the reaction is reversible. For this solution, the concentration of acid molecules, HCOOH, remains close to the solution molarity of 0.010 M because so few molecules dissociate. The H+ concentration is only 0.0013 M. Therefore, for the same solution molarity, the pH of the HCl solution is lower than the pH of the HCOOH solution.
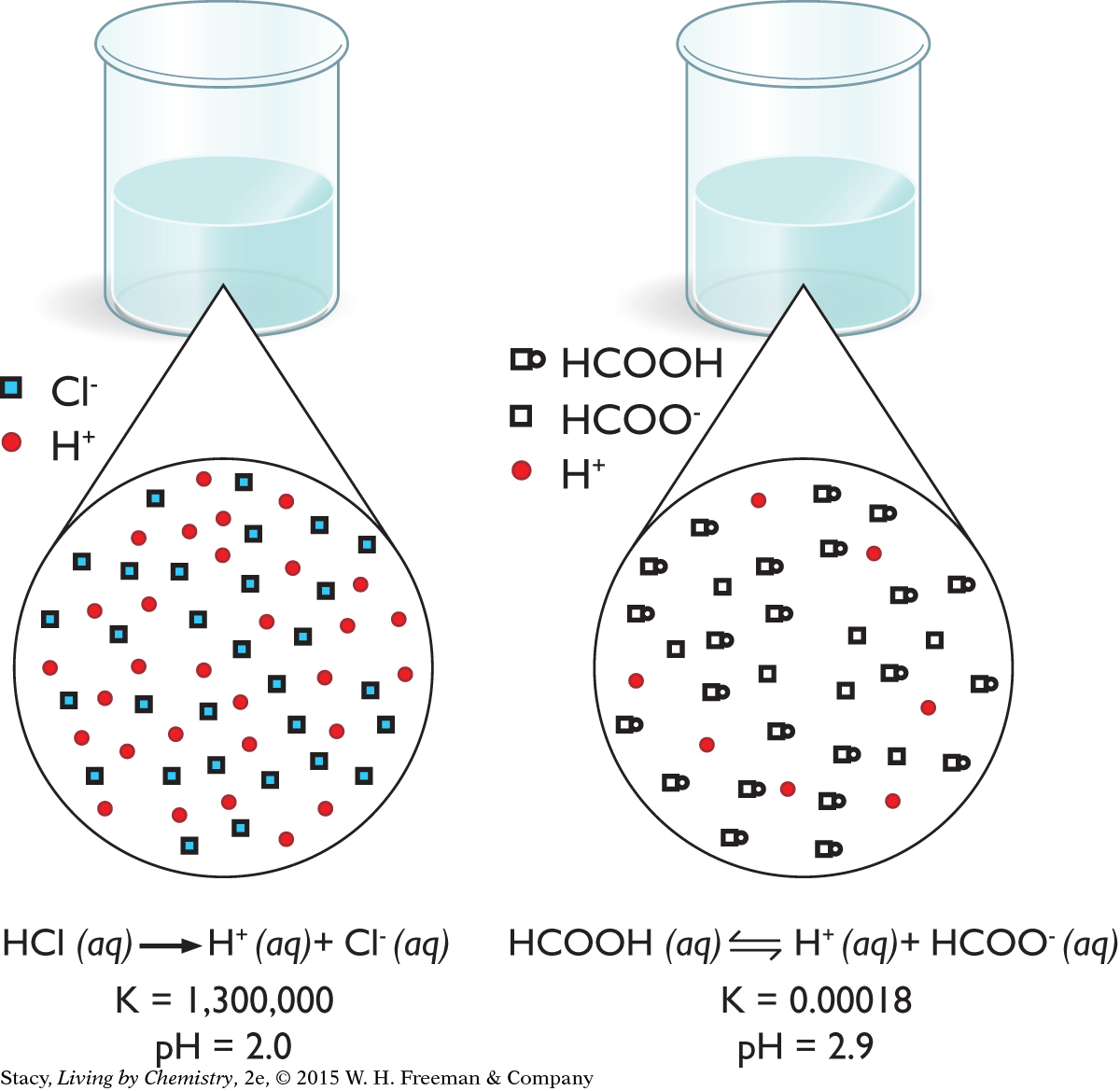
Important to Know
The word acid refers to undissociated acid molecules as well as to H+ dissolved in water. However, pH is only a measure of the H+ concentration in solution.
Example 2
The Value of K
A student prepares two aqueous solutions with a total acid concentration of 0.10 M. One solution is prepared with chlorous acid, HOClO, and the other with hypochlorous acid, HOCl. The equilibrium constant for HOClO is K = 0.011. The equilibrium constant for HOCl is K = 0.000000029. Which solution will have a lower pH value? Explain your thinking.
Solution
A larger value of K indicates more products and a greater degree of dissociation. This means that there will be more H+ in the HOClO solution, and the pH will be lower.
LESSON SUMMARY
LESSON SUMMARY
How can you predict if products are favored in a reversible process?
KEY TERM
equilibrium constant K
629
Chemical processes are characterized by an equilibrium constant K. The value of K is a measure of the degree to which the starting substances are converted to products. A large value of K indicates a large amount of product in the equilibrium mixture. A small value of K indicates very little product in the equilibrium mixture. Strong acids have a value of K that is very large, so products are favored. This means that very nearly all of the molecules are dissociated into ions. Weak acids have a value of K that is very small, which means that reactants are favored. This means that at any given time, only a small number of molecules are dissociated. The equilibrium constant K provides information about what is present in the solution and whether starting substances or products are favored in reversible processes.
Exercises
Reading Questions
Why is it useful to know the value of the equilibrium constant K for a reversible process?
How does the equilibrium constant K distinguish between strong and weak acids?
Reason and Apply
Hemoglobin is a large molecule in red blood cells that transports O2 from the lungs to cells in the human body. Consider the two reversible processes shown below involving oxygen, O2, and carbon monoxide, CO, attaching to hemoglobin.
hemoglobin + O2 ⇋ [hemoglobin : O2]
hemoglobin + CO ⇋ [hemoglobin : CO]
The binding of CO to hemoglobin is more than 200 times greater than the binding of O2 to hemoglobin.
Which reversible process has the larger equilibrium constant? Explain your thinking.
Sketch a particle view for both processes.
Explain why CO is extremely toxic to humans.
630
Use the table to answer the questions below. The brackets, [ ], indicate concentrations in the equilibrium mixture.
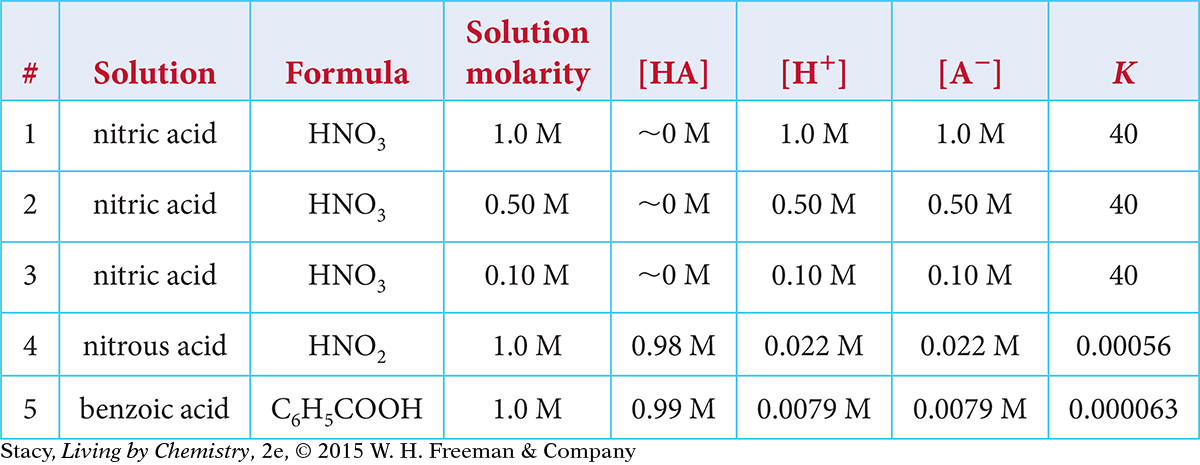
Describe how the H+concentration for Solutions 1, 2, and 3 relates to the solution molarity.
Explain how the value of K depends on the specific acid and the solution molarity.
Explain why [H+] = [A−].
For formic acid and acetic acid, describe a particle view of the equilibrium mixture.
Determine the pH for all five solutions.
If spilled, Solution 3 will cause more harm than Solution 5. Use this information to explain why pH, as opposed to molarity, is important in determining how dangerous an acidic solution is.