LESSON 122: How Pushy
THINK ABOUT IT
640
Human blood has a certain pH. When people exercise, they produce a lot of carbon dioxide, which in turn builds up carbonic acid in their bodies. A build-up of carbonic acid can alter the blood’s pH, which can put stress on the body. How does the body maintain a healthy equilibrium?
What happens to a system at equilibrium when conditions change?
To answer this question, you will explore
Changing Conditions
Le Châtelier’s Principle
Changing Conditions
EXPLORING THE TOPIC
Changing Conditions
When a mixture is at equilibrium, the forward and reverse processes are dynamically balanced, going backward and forward at equal rates. For example, if an indicator is dissolved in water, the indicator, HIn, dissociates in the forward process and the ions, H+ and In−, attract one another in the reverse process. Although equilibrium is dynamic, once equilibrium is established, the concentrations of HIn, H+, and In− in the aqueous solution do not change over time, as long as the conditions stay the same.
However, conditions do not always stay the same for an equilibrium mixture. Something new might be added to the mixture or the mixture might be heated or cooled. For example, HCl might be added to the indicator solution described above. Such changes disturb the equilibrium, which changes in a predictable way by adjusting to new concentrations to counteract the disturbance.
Here are three types of changes that can be made to an equilibrium mixture:
A change in the concentrations—this applies when more of the starting substances or products are added to an equilibrium mixture.
A change in external pressure—this applies to processes involving gases when the container volume is increased or decreased.
A change in temperature—heating or cooling an equilibrium mixture changes the value of K.
Changing the conditions for an equilibrium mixture will disturb the system. The examples here demonstrate how each of the three types of changes (or disturbances) affects a reversible process at equilibrium.
COBALT CHLORIDE EQUILIBRIA
641
The dissociation of tetrachlorocobalt (II) ions, CoCl42−(aq), to aqueous cobalt (II) ions, Co2+(aq) and chloride ions, Cl−(aq), is especially useful in demonstrating changes in equilibrium because it involves a striking color change.
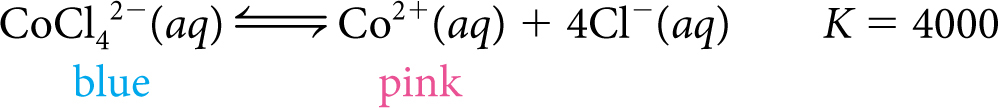
Cobalt chloride solutions can be pink, blue, or various shades of purple. This figure shows molecular views of tiny volumes of a pink and blue cobalt solution. Notice that there are more Co2+ ions (pink circles) than CoCl42− ions (blue circles) in the pink solution, but more CoCl42– ions in the blue solution. Notice also that the blue solution has a larger number of Cl− ions (gray circles).
Important to Know
Equilibrium mixtures can have various concentrations of starting substance and products. For example, equilibrium mixtures of cobalt chloride can be pink, blue, or various shades of purple.
It is possible to predict how a disturbance, such as the addition of Cl− ions to the pink cobalt solution, will change the equilibrium mixture.
CHANGES IN CONCENTRATION
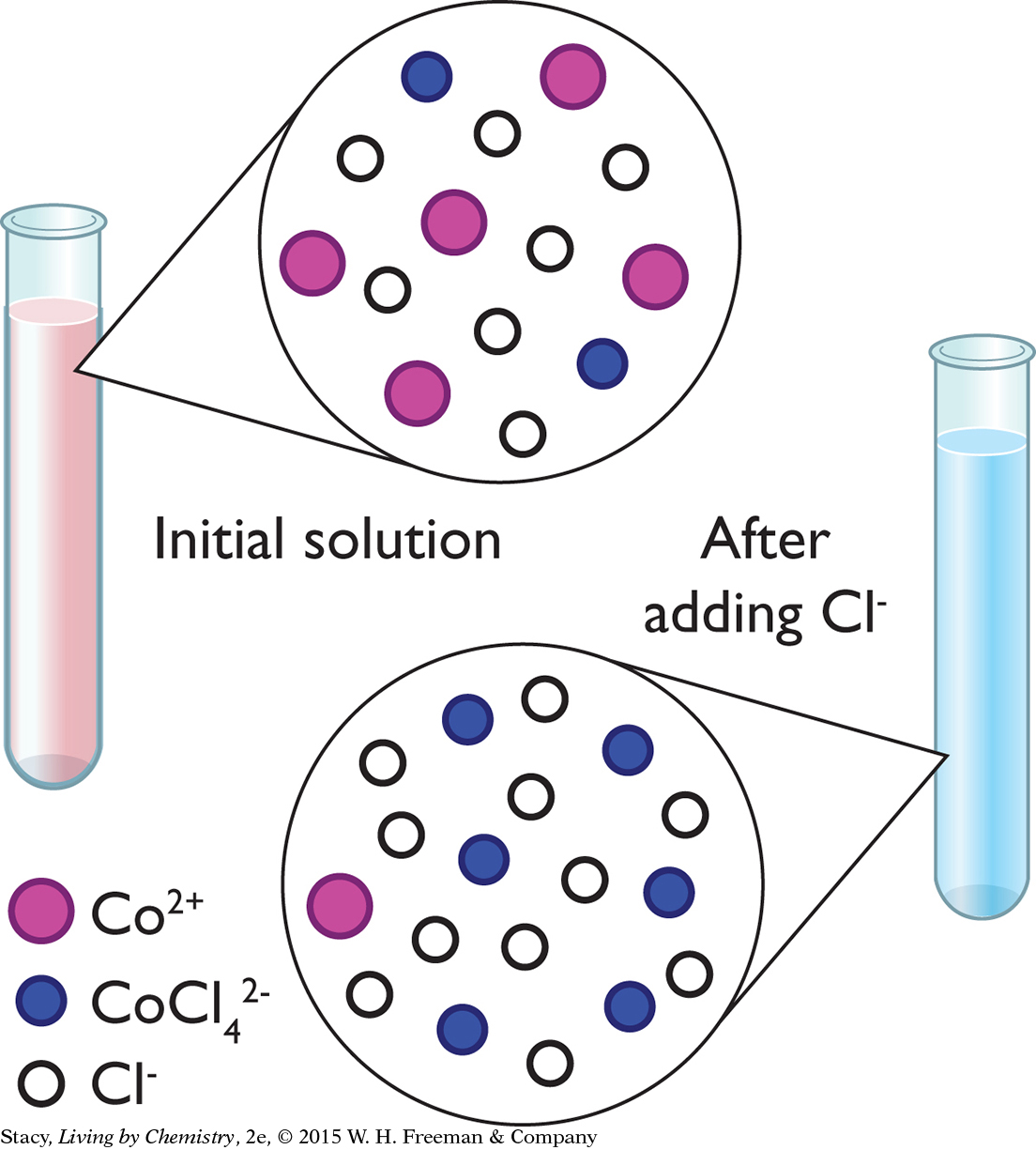
Imagine that you have a pink sample of a cobalt chloride solution. The pink color indicates that the sample has more Co2+ ions (one of the products) than CoCl42− ions (starting substance). If you change the conditions by adding more Cl− ions to the mixture, the sample turns blue. So adding more products results in the formation of more starting substance.
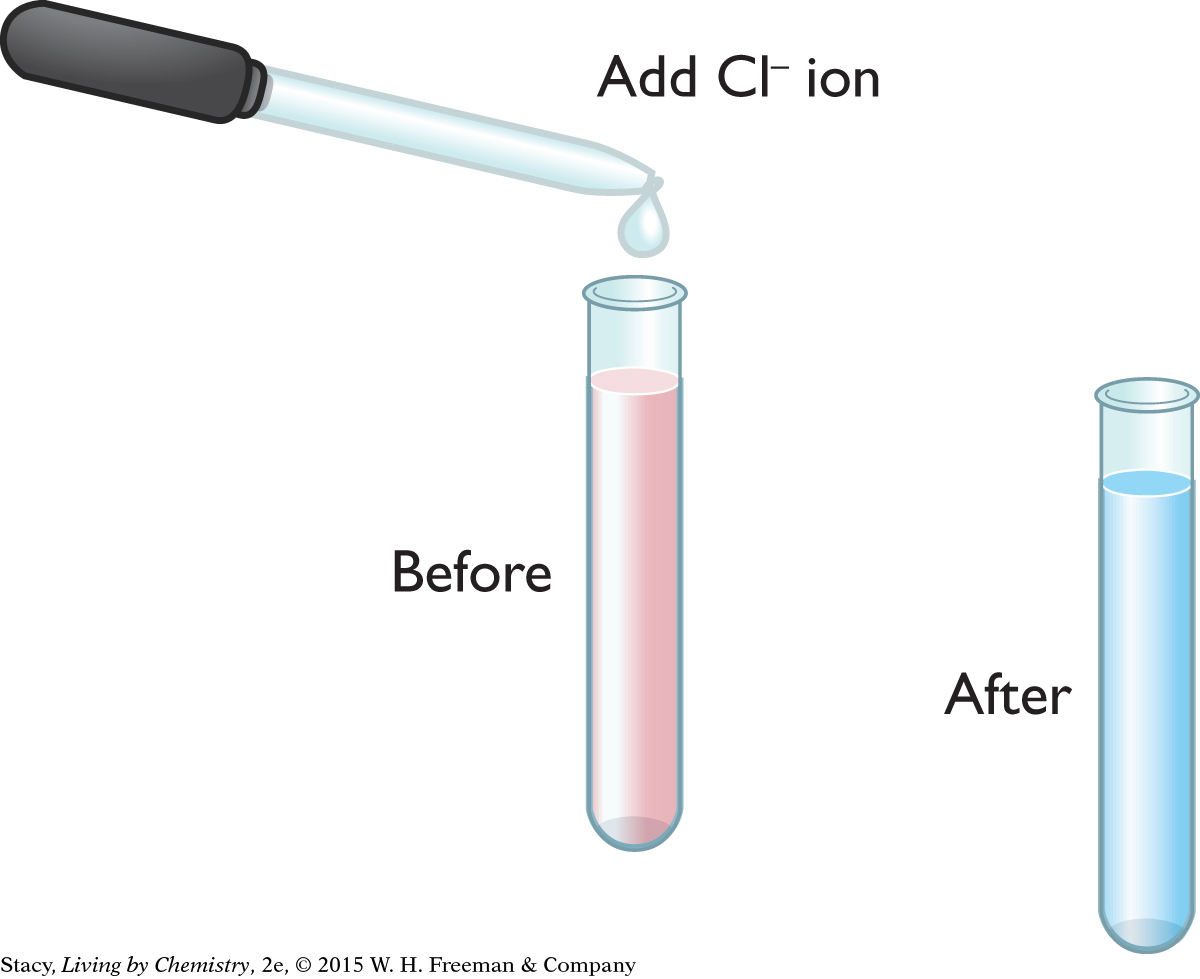
If you take this blue sample and add silver ions, AgNO3(aq), to remove chloride ions, Cl−, by precipitating AgCl(s), the mixture changes back to pink. So removing Cl− ions (one of the products) results in the formation of more products (Co2+ and Cl− ions). The illustration shows the results of these two changes.
642
The color changes indicate that adding or removing one of the products in an equilibrium mixture has the effect of changing the final concentrations of the new equilibrium mixture. Likewise, the equilibrium mixture also changes if the concentration of starting substance is changed. The change depends on which side of the reversible process gets the stress.
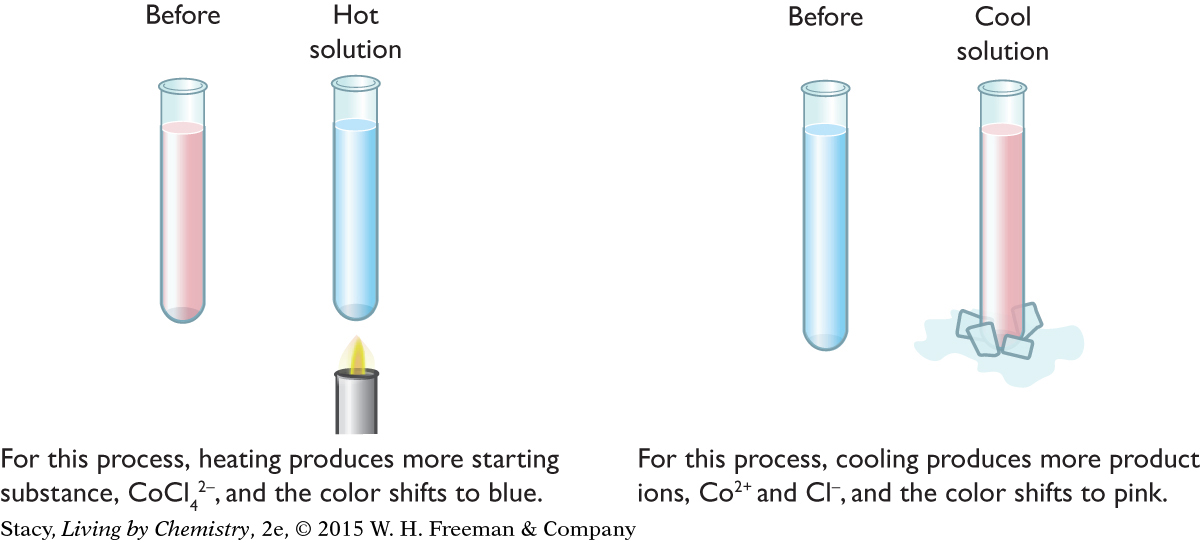
CHANGES IN TEMPERATURE
This same reversible process can be used to test the effects of changing temperatures on an equilibrium mixture. If a pink starting sample of cobalt chloride solution is heated, it turns blue. If a blue sample of this mixture is cooled, it turns back to pink or pinkish-purple. In this particular reversible process, heating favors the starting substance.
Important to Know
Changes in temperature change the value of K. Other changes, such as changes in pressure or concentration, stress the system, but they do not change the value of K.
Heating does not always cause more starting substance to form. The direction of the change depends on whether the process is exothermic or endothermic. For an endothermic process, heating produces more products. For an exothermic process, cooling produces more products. The reversible cobalt process is exothermic in the directions the chemical equation is written, so heating causes the formation of more starting substance.
CHANGES IN PRESSURE
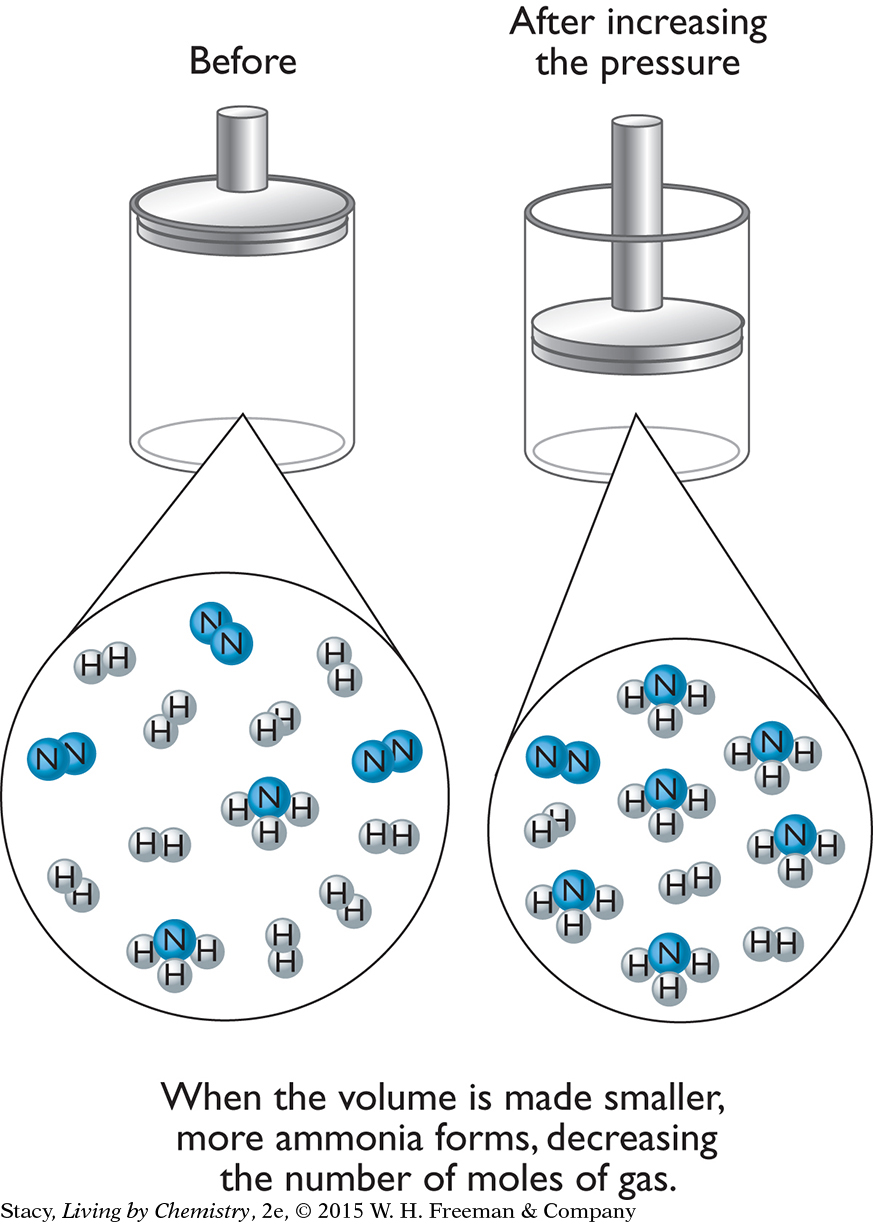
Pressure changes are particularly important when considering equilibrium mixtures that are in the gas phase. Recall from Unit 3: Weather that it is common to use a cylinder and piston as a flexible container for a gas. Pushing down on the piston to decrease the gas volume increases the external pressure on a gaseous equilibrium mixture. A change will occur in the direction that decreases the number of moles of gas and relieves some of the pressure. Recall that the pressure decreases as the number of moles decreases for the same temperature and volume.
643
For example, consider the process for the formation of ammonia gas from nitrogen and hydrogen gases.
N2(g) + 3H2(g) ⇋ 2NH3(g)
Decreasing the volume of the container increases the pressure on the gas mixture. The system will change so that more ammonia forms. This is because there are four moles of gas on the starting substance side of the equation and only two moles of gas on the product side.
The creation of more ammonia relieves the stress of the added pressure by reducing the total number of moles of gas in the mixture.
Le Châtelier’s Principle
Le Châtelier’s Principle
These very predictable changes to equilibrium mixtures were first noted around 1885 by a French chemist named Henry Le Châtelier. He observed that when the conditions of an equilibrium mixture are modified, the system responds in such a way as to reduce the effect of the change. This idea is known as Le Châtelier’s Principle.
Le Châtelier’s Principle
When conditions change for a system at equilibrium, the system will respond by reducing the effect of the change.
WEATHER CONNECTION
WEATHER
CONNECTION
On a humid day, there is a lot of water vapor in the air. When you exercise in humid weather, your sweat does not evaporate because the air is already nearly saturated with water vapor. Beads of sweat accumulate on your body. In contrast, when you exercise in a dry climate, you lose a lot of water because your sweat evaporates. This is why it is especially important to drink a lot of water in dry climates.

Le Châtelier’s principle allows you to predict qualitatively how the system responds to changes in concentration, pressure, or temperature.
AN EXPLANATION OF LE CHÂTELIER’S PRINCIPLE
If more of one of the products is added to the equilibrium mixture of starting substances and products, this momentarily increases the rate of the reverse process. The concentrations in the mixture adjust until the rates of the forward and reverse processes are equal. This adjustment results in a new equilibrium mixture that has more starting substance compared with that of the initial solution. The value of the equilibrium constant K is the same for both the adjusted mixture and the initial solution.
A second change is to raise or lower the solution’s temperature, which also changes the composition of the equilibrium mixture of starting substances and products. In this case, the changes in composition result in a new value of K depending on if the forward process is exothermic or endothermic. Because there is a new value of K, the concentrations of starting substances and products change to satisfy the new equilibrium constant equation. If the forward process is exothermic (heat is released), the value of K gets smaller as the temperature is raised. Likewise, if the forward process is endothermic (heat is required), the value of K gets larger as the temperature is raised.
MEDICAL CONNECTION
MEDICAL
CONNECTION
Circulatory shock occurs when there is an injury that causes extensive bleeding. As a consequence, cells in the human body do not get enough oxygen due to low blood flow. Instead of converting glucose to carbon dioxide and water, cells produce lactic acid. This creates a condition called acidosis. In certain circumstances, an injection of sodium bicarbonate (baking soda) into the bloodstream lowers the acidity of the blood, and raises the blood’s pH back to normal.

644
BLOOD pH AND EXERCISE
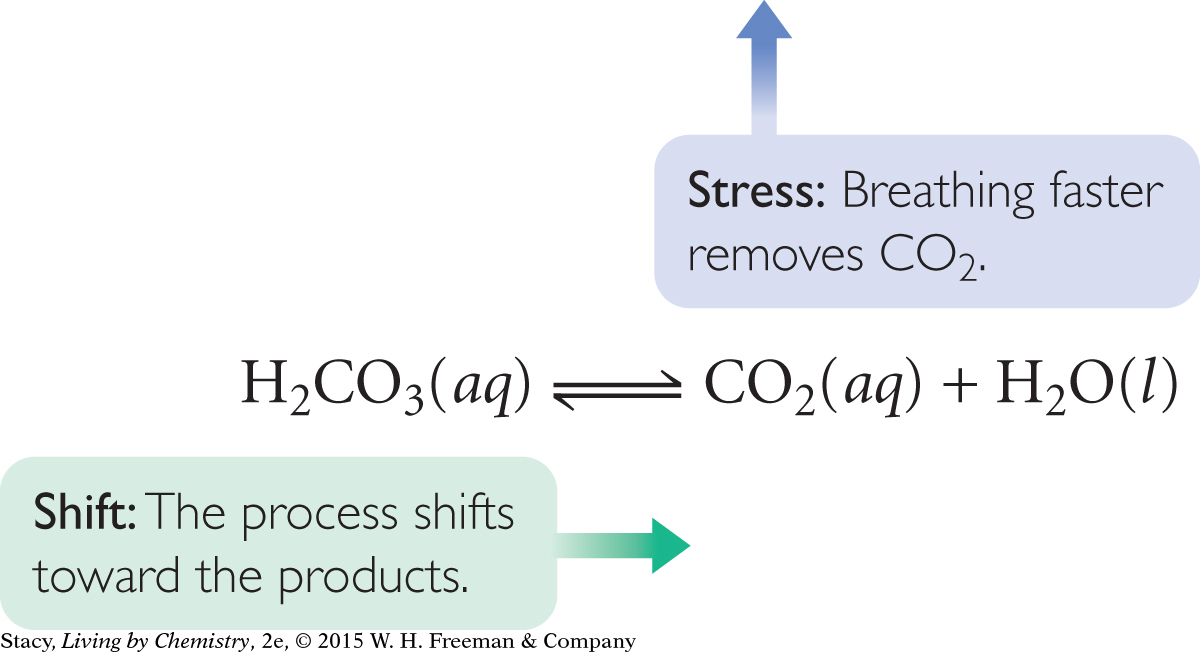
The human body is a large collection of reversible processes. For processes to proceed at the appropriate rates, and for substances to stay in the appropriate concentrations within our bodies, a balance must be maintained. For example, the pH of the body’s blood is normally around 7.4, slightly basic. When you exercise, more carbon dioxide is produced, and it dissolves in the bloodstream. This is a natural waste product that you normally exhale from the lungs. However, carbon dioxide in the bloodstream also leads to the formation of carbonic acid, H2CO3. A large build up of carbonic acid would increase the acidity of the blood and lower the pH.
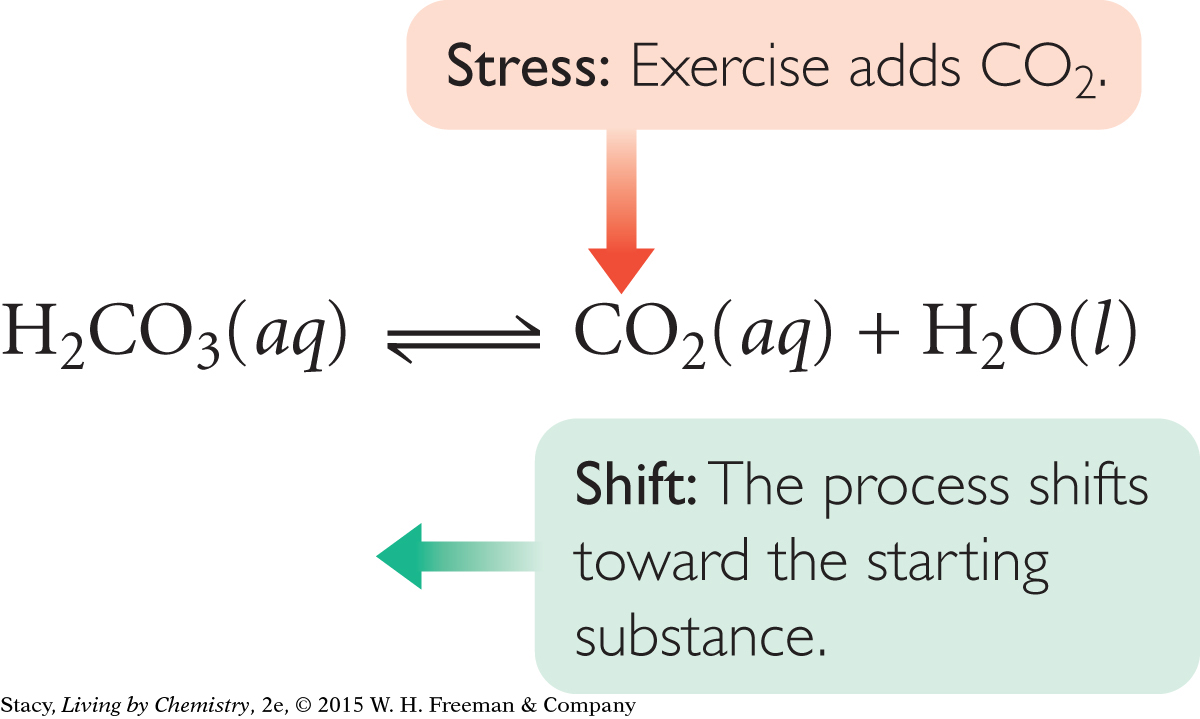
One of the ways your body deals with a change in the blood’s pH is to exhale more rapidly, removing CO2 from the bloodstream and shifting the process back toward the right. This is one reason why we breathe more heavily when exercising—to rid the body of excess carbon dioxide and increase the blood’s pH.

Example
Decomposition of SO3
Gaseous sulfur trioxide, SO3, decomposes to form sulfur dioxide, SO2, and oxygen, O2. The process is endothermic. Predict how the changes listed here will affect the process.
2SO3(g) ⇋ 2SO2(g) + O2(g)
Increasing the pressure on the system
Adding more O2
Removing O2 from the system
Adding heat
Solution
In the forward process, two moles of gas decompose to form three moles of gas. The system can minimize the effect of a pressure increase by converting some of the products back to SO3 to decrease the number of moles of gas molecules present. This will push the process more toward the starting substances.
645
Adding more O2 to the system will cause more starting substance to form. By making more SO3, some of the excess O2 is removed, relieving the stress on the system.
Removing O2 from the system has the opposite effect; it causes the formation of more products. This change has the effect of restoring some of the O2 that was removed.
Because energy is absorbed in the forward process, heating will favor products.
LESSON SUMMARY
LESSON SUMMARY
What happens to a system at equilibrium when conditions change?
KEY TERM
Le Châtelier’s principle
When conditions are changed for a system at equilibrium, the system will respond by reducing the effect of the change. This is known as Le Châtelier’s principle. Changing conditions (or stresses) include changes to temperature, concentration, or pressure. A system regains equilibrium by rebalancing the concentrations of starting substances and products. The exact nature of the effects on a system are predictable because they depend on the type of change in condition that the system encounters. A temperature change affects the value of K. A pressure change or a change in concentration stresses the system, but does not change the value of K.
Exercises
Reading Questions
Name two things that you can do to form more starting substances in an equilibrium mixture.
Based on the evidence provided in this lesson, explain how you can tell if a process is exothermic as written.
Reason and Apply
If you add more ammonia, NH3(aq), to the equilibrium mixture described by the chemical equation given below, what will happen? Explain your thinking.
Cu(NH3)62+(aq) ⇋ Cu2+(aq) + 6NH3(aq)
If you remove ammonia, NH3(g), from the equilibrium mixture described by the equation here, what will happen? Explain your thinking.
N2(g) + 3H2(g) ⇋ 2NH3(g)
If you heat the equilibrium mixture for this endothermic process, what will happen? Explain your thinking.
2NiO(s) ⇋ 2Ni(s) + O2(g)
Suppose that you want to dissolve more copper (II) hydroxide, Cu(OH)2(s), in an aqueous solution. Would you add acid or base? Explain your thinking.
Cu(OH)2(s) ⇋ Cu2+(aq) + 2OH−(aq)
For each reversible process listed, describe two things you can do to change the concentration of one of the products.
2NO2(g) ⇋ N2O4(g)
HF(aq) ⇋ H+(aq) + F(aq)
PbCl2(s) ⇋ Pb2+(aq) + 2Cl−(aq)
CaCO3(s) ⇋ CaO(s) + CO2(g)