LESSON 123: How Colorful
646
THINK ABOUT IT
Acid-base indicators, such as red cabbage juice, change color as the H+ concentration in a solution is changed. The pH at which the color changes is different for different indictors. For example, cabbage juice changes color from red to blue at pH ~7, whereas phenolphthalein changes color from colorless to pink at pH ~9. How do equilibrium considerations predict the pH for the color change?
How do acid-base indicators work?
To answer this question, you will explore
Applying Le Châtelier’s Principle to Acid-Base Indicator Solutions
Relating the Value of K and the pH to the Indicator Color
Types of Equilibrium Constants
Applying Le Châtelier’s Principle to Acid-Base Indicator Solutions
EXPLORING THE TOPIC
Applying Le Châtelier’s Principle to Acid-Base Indicator Solutions
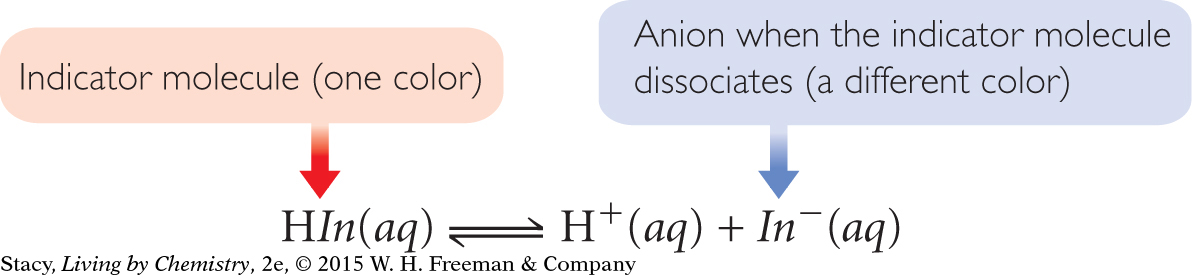
Acid-base indicators are weak acids, chosen for the bright colors of the HIn molecule and In− ion. Like other weak acids, the dissociation process of the indicator molecules to H+ and an anion in aqueous solutions is reversible.
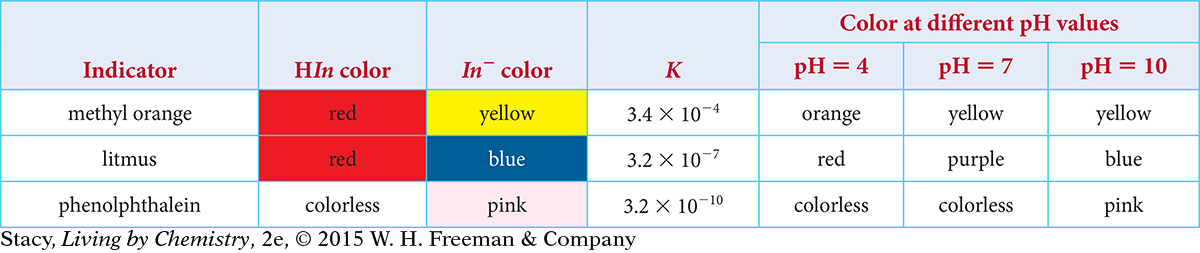
COLORS AT DIFFERENT pH VALUES
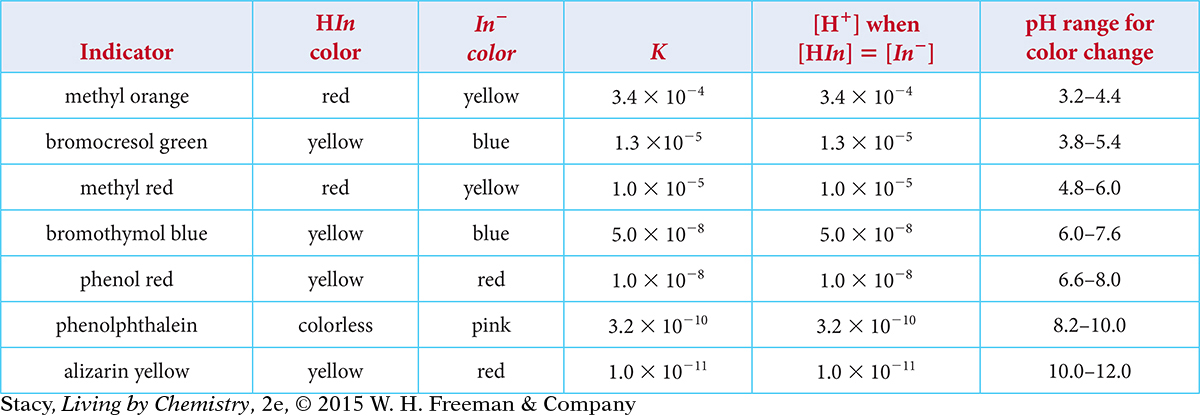
Each indicator has a specific value for the equilibrium constant K and a certain color that depends on the pH of the solution. Examine the information in the table to look for a relationship between K, pH, and color.
Here are some things you might notice:
HISTORY CONNECTION
HISTORY
CONNECTION
Litmus is a mixture of water-soluble organic compounds that are extracted from certain lichens. Often this mixture is infused into filter paper to make litmus paper. Lichens themselves are species of fungus that exist in a close symbiotic relationship with species of photosynthetic algae.

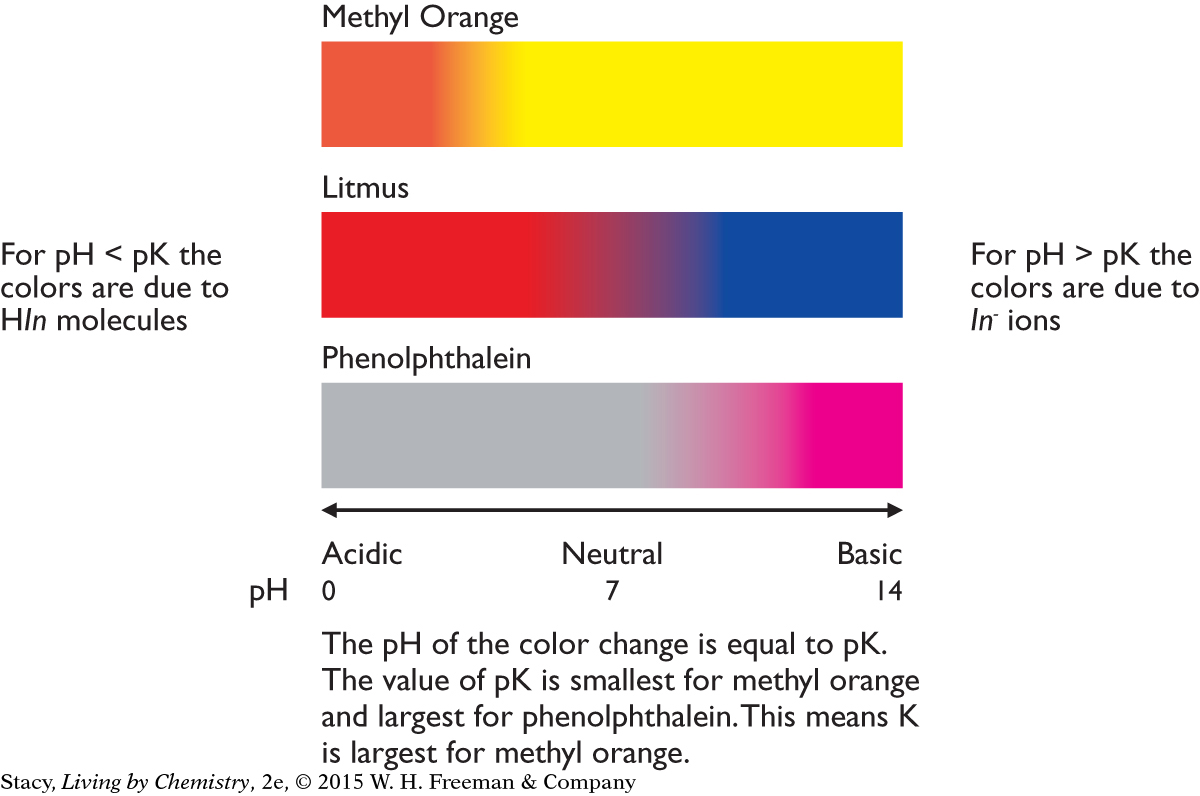
The value of K: The equilibrium constants are all much less than 1, indicating that the concentrations of the products are quite small. This is because these indicators are very weak acids that do not dissociate very much. The value of K for methyl orange is the largest, so, of these three indicators, it dissociates the most. Phenolphthalein, with the smallest K value, dissociates the least. The value of K for litmus is in between the values for methyl orange and phenolphthalein.
647
The color at pH = 7: A methyl orange solution is yellow at pH = 7, showing that the yellow In− ion is present in relatively large concentration. A phenolphthalein solution is colorless, showing that the colorless HIn molecules is present in relatively large concentration. A litmus solution, being purple, is a mixture of red HIn molecules and blue In− ions in roughly equal concentrations. Notice that the methyl orange solution, with the largest value of K, has the most product ions. The phenolphthalein solution has the smallest value of K and the least number of product ions.
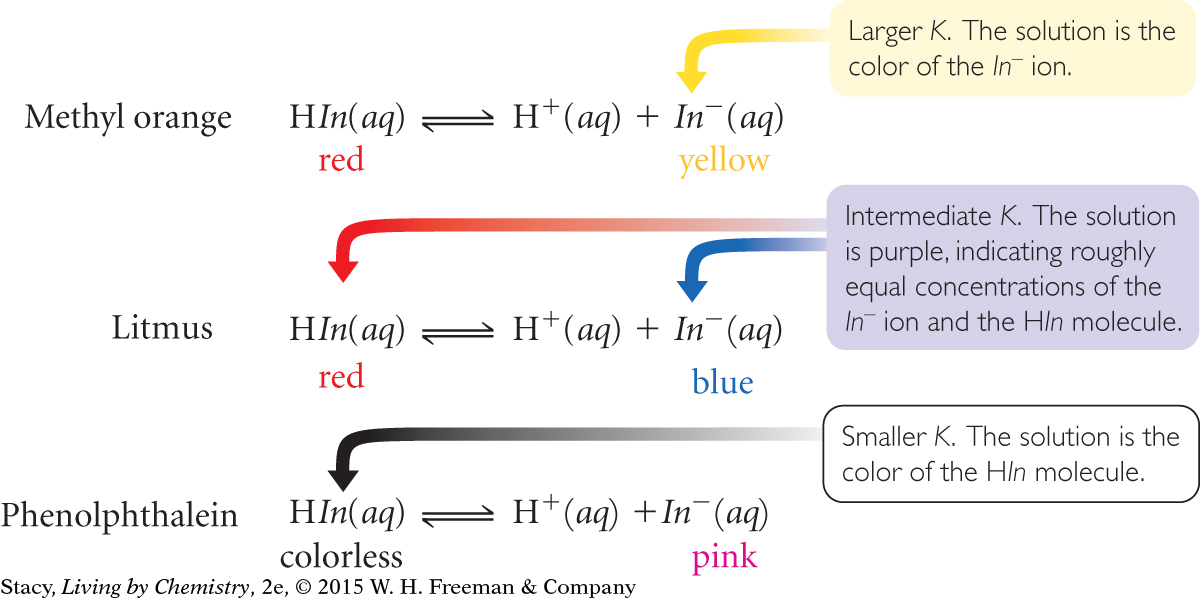
The color at different pH values: As the pH value increases, the solution color changes from the color of HIn in acidic solutions to the color of In− in basic solutions. The illustration below shows the color changes for the three indicators in the table. Notice that the color change occurs at higher pH values as the equilibrium constant K gets smaller.
MINIMIZING CHANGES AT EQUILIBRIUM
Important to Know
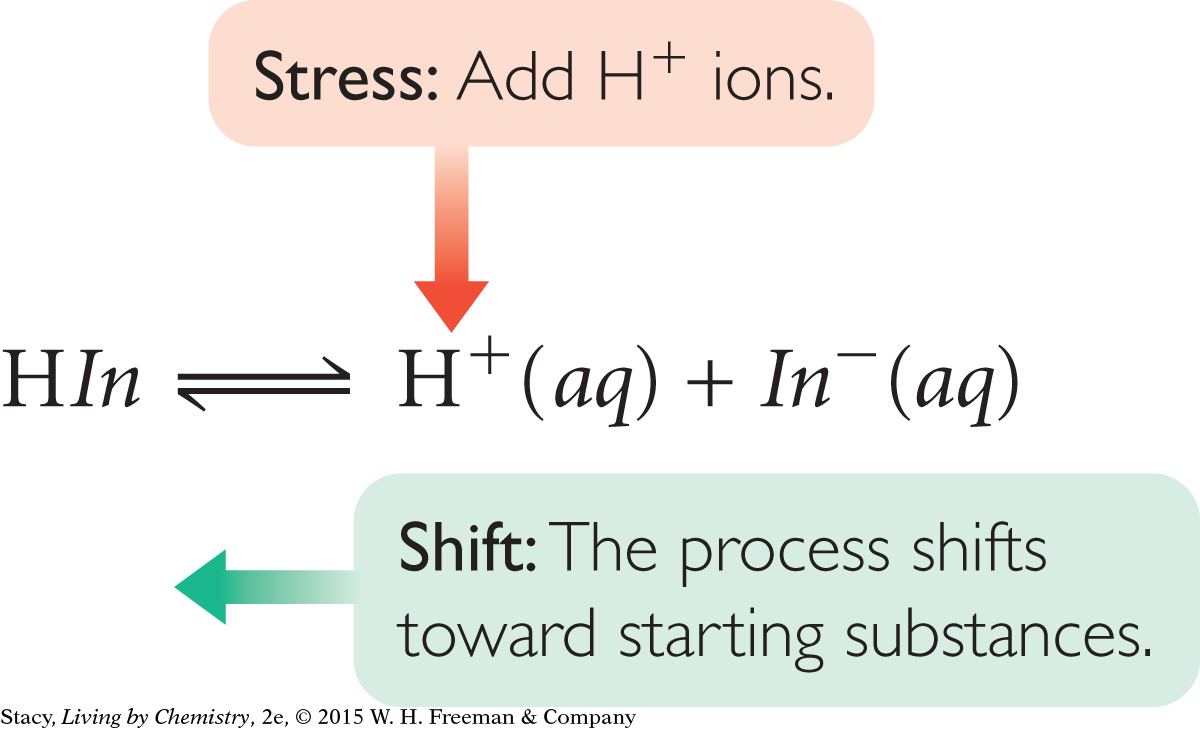
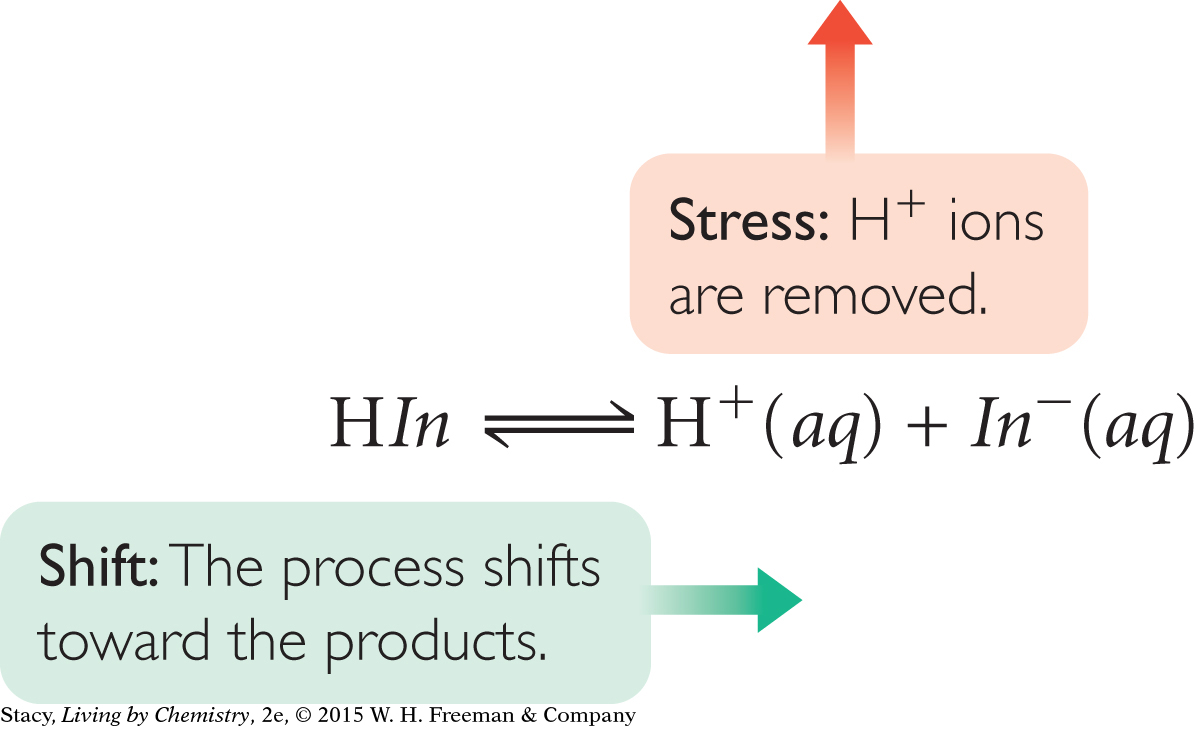
For these acid-base indicator solutions, an acid or base is added to change the value of the pH. For this reason, the concentration of H+ is not equal to the concentration of In−.
648
One way to understand the color differences for indicators in acidic and basic solutions is to apply Le Châtelier’s principle. When an acid is added to a solution with an indicator, [H+] increases. Because H+ is a product of the dissociation of the indicator, the system responds to minimize the change by forming HIn molecules. Alternatively, if a base is added to a solution with an indicator, [H+] decreases. The system responds by making more product ions, H+ and In−.
|
|
Relating the Value of K and the pH to the Indicator Color
Relating the Value of K and the pH to the Indicator Color
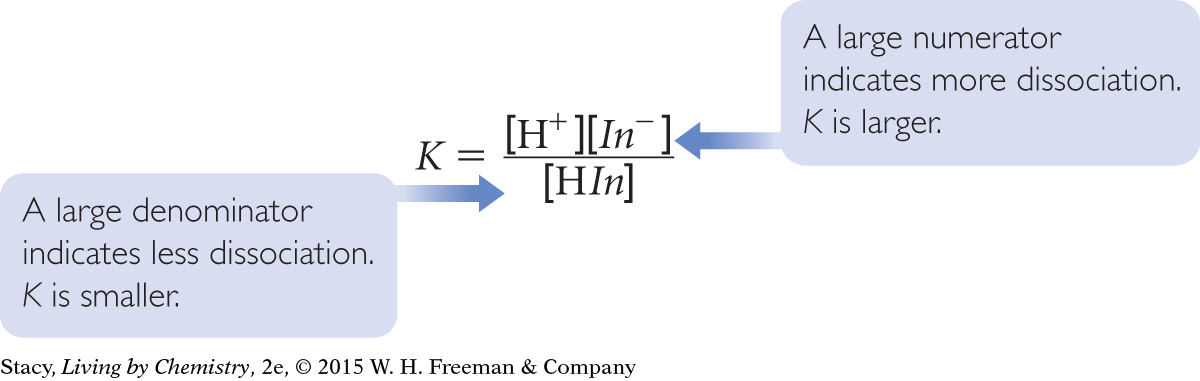
The color change of the indicator signals the shift that has occurred in the mixture to minimize the changes that occur. This can also be seen mathematically if you examine the equilibrium-constant equation.
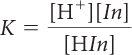
Important to Know
In pure water, [H+] = [In−]. However, this is no longer true if a different acid or base is added to change [H+].
Since K is a constant, it does not change. If any variable in the equation changes, the other variables must adjust. For example, if [H+] increases, [In−] must decrease and [HIn] must increase to keep K the same.
pH RANGE FOR COLOR CHANGE
Take a moment to examine the information for the color change for several indicators in the table. Notice that the color change occurs at different pH values for different indicators.
649
The color change for an indicator occurs when the H+ ion concentration is equal to K. This happens when the concentration of HIn molecules is equal to the concentration of the In− ions. Rearranging the equilibrium-constant equation proves this to be true.
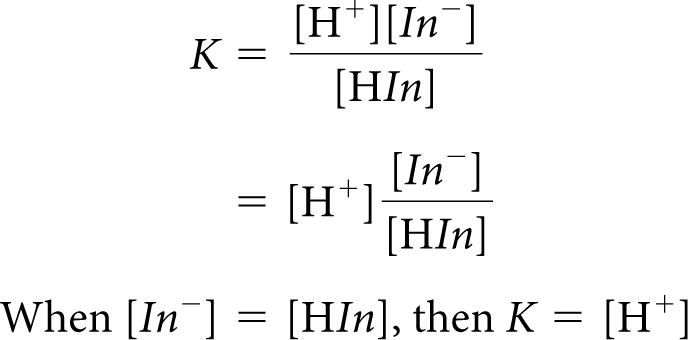
When [H+] > K, then [HIn] > [In−]. The solution has the color of HIn molecules. When [H+] < K, then [HIn] < [In−]. The solution has the color of In− ions. When [H+] = K, then [HIn] = [In−]. The solution color is a mixture of the color of HIn molecules and In− ions.
Example 1
The Color of Congo Red
Determine the pH value at which the indicator Congo red changes color. The equilibrium constant for the dissociation of the indicator is K = 10−4.
Solution
The color change happens when the concentration of HIn equals the concentration of In−. If [HIn] = [In−], then K = [H+] = 10−4.
pH = −log[H+]
= −log[10−4]
= 4
So the indicator changes color at pH = 4.
Types of Equilibrium Constants
Types of Equilibrium Constants
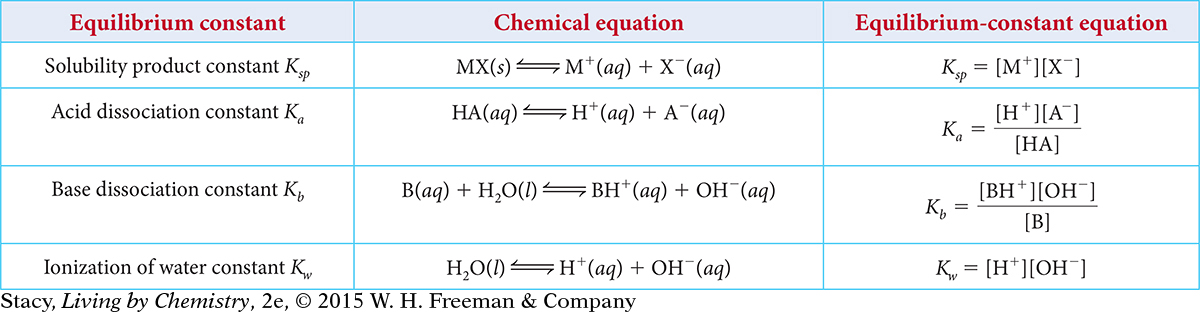
The equilibrium constant K can be used to solve a variety of equilibrium problems. In every case, the equilibrium-constant equation can be used to figure out the concentrations of one or more of the compounds in the equilibrium mixture. The table shows some specific types of chemical equilibrium constants. Each of these constants is designated by a unique subscript.
650
Notice that the equilibrium-constant equations are the same as those that you might have written, except that they have a subscript attached to the symbol K. As discussed previously, liquids and solids are not included in the equilibrium-constant equation because their concentrations do not change. So, equilibrium-constant equations can be used to determine the concentrations of molecules and ions in solution and the concentrations of gaseous molecules in systems at equilibrium.
The equilibrium constant Ksp can be used to determine the solubility of an ionic compound.
Example 2
Comparing Solubility
The solubility product constants Ksp for several alkaline earth metal sulfates at 25 ºC are given in the table.
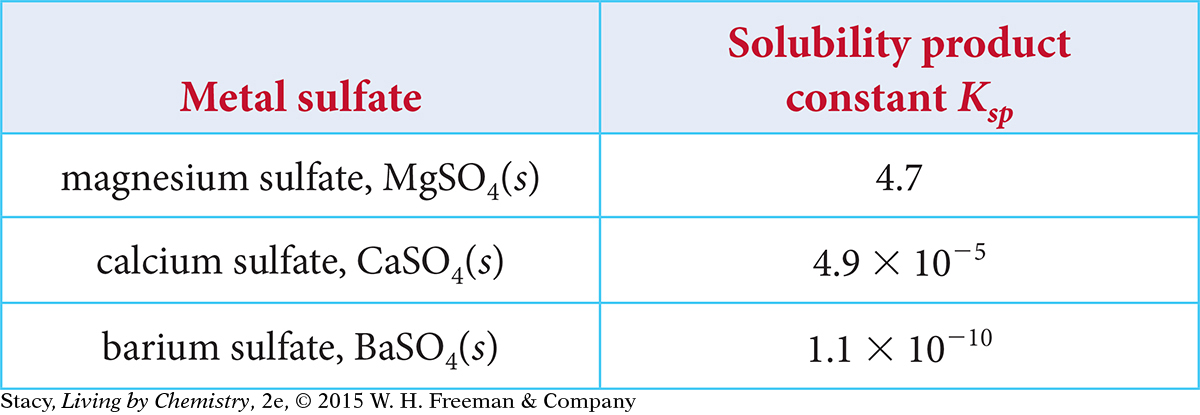
Put the three salts in order of increasing solubility from least soluble to most soluble. Explain your thinking.
Use the periodic table to predict whether strontium sulfate, SrSO4(s), is more soluble than calcium sulfate, CaSO4(s). Explain your thinking.
Suppose you add sodium sulfate, Na2SO4(s), to a saturated solution of calcium sulfate, CaSO4(aq). Use Le Châtelier’s principle to explain why a small amount of CaSO4(s) precipitates from the solution.
Solution
The chemical equation for the solubility of an alkaline earth metal sulfate, MSO4(s), is
MSO4(s) ⇋ M2+(aq) + SO42−(aq) Ksp = [M2+][SO42−]
If the solubility product constant Ksp is large, products are favored (the salt is more soluble). Therefore, the solubility from least soluble to most soluble is BaSO4 < CaSO4 < MgSO4.
All of the metals are in Group 2A, the alkaline earth metals. The solubility decreases as you go down the group. So, you expect strontium sulfate, SrSO4(s), to be less soluble than calcium sulfate, CaSO4(s), but more soluble than barium sulfate, BaSO4(s).
Adding sodium sulfate, Na2SO4(s), adds more sulfate ions. Because SO42−(aq) is a product of dissolving calcium sulfate, CaSO4(aq), the system will reduce the stress by forming more reactants. This is why some CaSO4(s) precipitates.
651
The equilibrium constant Ka can be used to determine the strength of an acid or a base.
Example 3
Strength of an Acid
Imagine that you prepare a 0.10 M solution of HCl and a 0.10 M solution of HOCl. The concentrations of the ions in equilibrium are given in this table.
The general chemical equation for acid dissociation is:
HA(aq) ⇋ H+(aq) + A−(aq)
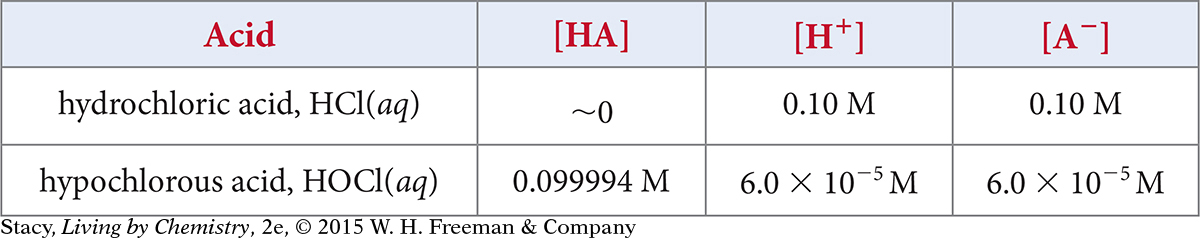
Determine Ka for hypochlorous acid, HOCl(aq).
Explain why Ka for HCl(aq) is a very large number.
Which acid is stronger? Explain your thinking.
Solution
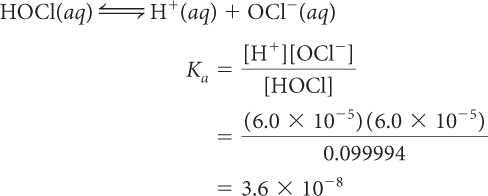
The value [HCl] is extremely small since it is approximately zero. Dividing by such a small number gives a very large number, so K for HCl must be extremely large.
So Ka for this acid is very large.
The very large Ka for HCl indicates that HCl fully dissociates in water. The tiny Ka for HOCl indicates that HOCl does not dissociate as much as HCl. So HCl is a stronger acid than HOCl.
LESSON SUMMARY
LESSON SUMMARY
652
How do acid-base indicators work?
Acid-base indicators are weak acids or bases that change color depending on pH. The concentrations of an indicator molecule and its anion depend on the concentration of H+ ions in solution. Therefore, the color of an indicator is a measure of the acidity of the solution. The color of an indicator changes when the concentrations of the HIn molecules and In− anions are identical. This occurs when K = [H+]. The lessons learned from applying equilibrium ideas to indicators are general, and they can be applied to other types of equilibria.
Exercises
Reading Questions
Explain how an acid-base indicator works to show the pH of a solution.
Does the value of K for an indicator change when the indicator is added to an acid or a base solution? Explain.
Reason and Apply
The solubility product constants Kap at 25 ºC for several chlorides are provided in the table.
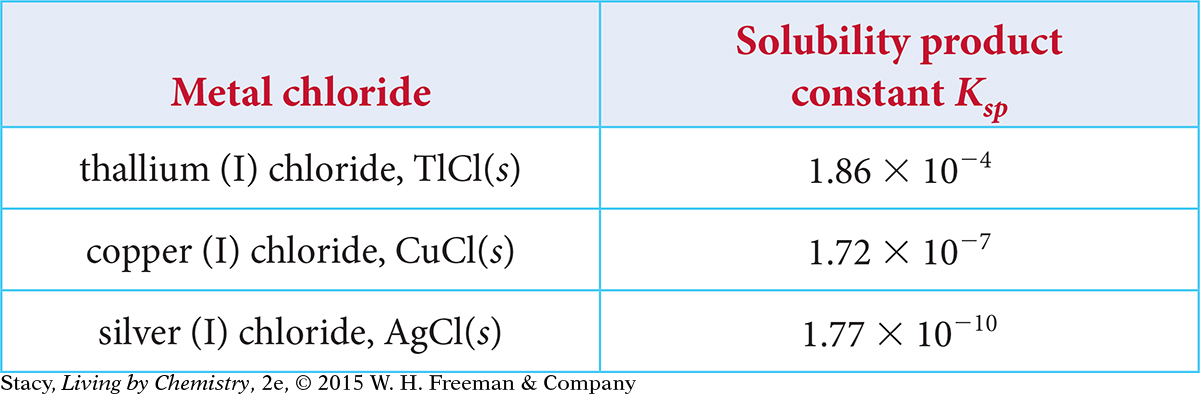
Put the three salts in order of increasing solubility—from least soluble to most soluble. Explain your thinking.
Suppose that you add sodium chloride, NaCl(s), to a saturated solution of copper chloride, CuCl(aq). Use Le Châtelier’s principle to explain what happens. Assume that NaCl is very soluble.
Imagine you prepare a 0.10 M solution of nitric acid, HNO3, and a 0.10 M solution of hydrofluoric acid, HF. The concentrations of the ions in equilibrium are given in the table.
General equation for acid dissociation: HA(aq) ⇋ H+(aq) +A−(aq)
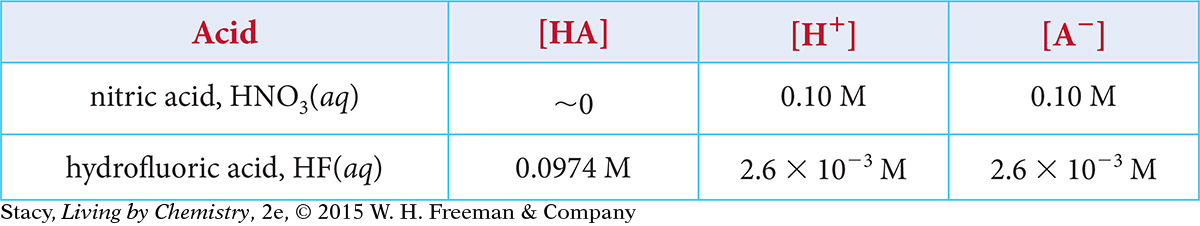
Determine K for hydrofluoric acid, HF.
Explain why K for nitric acid, HNO3, is a very large number.
Which acid is stronger? Explain your thinking.
Which solution has the higher concentration of H+(aq), 0.10 M HNO3 or 0.10 M HF? Explain your thinking.
653
Use the table on page 648 to predict the color of these indicators if you dissolve them in water. Explain your thinking.
alizarin yellow
bromocresol green
methyl red
phenol red
Use Le Châtelier’s principle to explain why bromocresol green is blue if you dissolve it in a base solution, such as 0.10 M sodium hydroxide, NaOH.
Determine the pH value at which thymolphthalein changes color. The equilibrium constant for the dissociation of the indicator is K = 10−10.
Methyl violet changes color from the yellow HIn molecule to the blue-violet In− ion at a pH of 1. What is the equilibrium constant for the dissociation of methyl violet?
Look up the source, structure, and properties of a particular indicator. Write a short description of the molecule.