LESSON 7: Now You See It: The Copper Cycle
27
THINK ABOUT IT
At this point, you are probably convinced that a copper penny cannot be transformed into real gold in a high-school chemistry classroom. However, the question remains, what happened to the copper penny?
What happens to matter when it is changed?
To answer this question, you will explore
The Copper Cycle
Evidence of Chemical Change
Interpreting Observations
The Copper Cycle
EXPLORING THE TOPIC
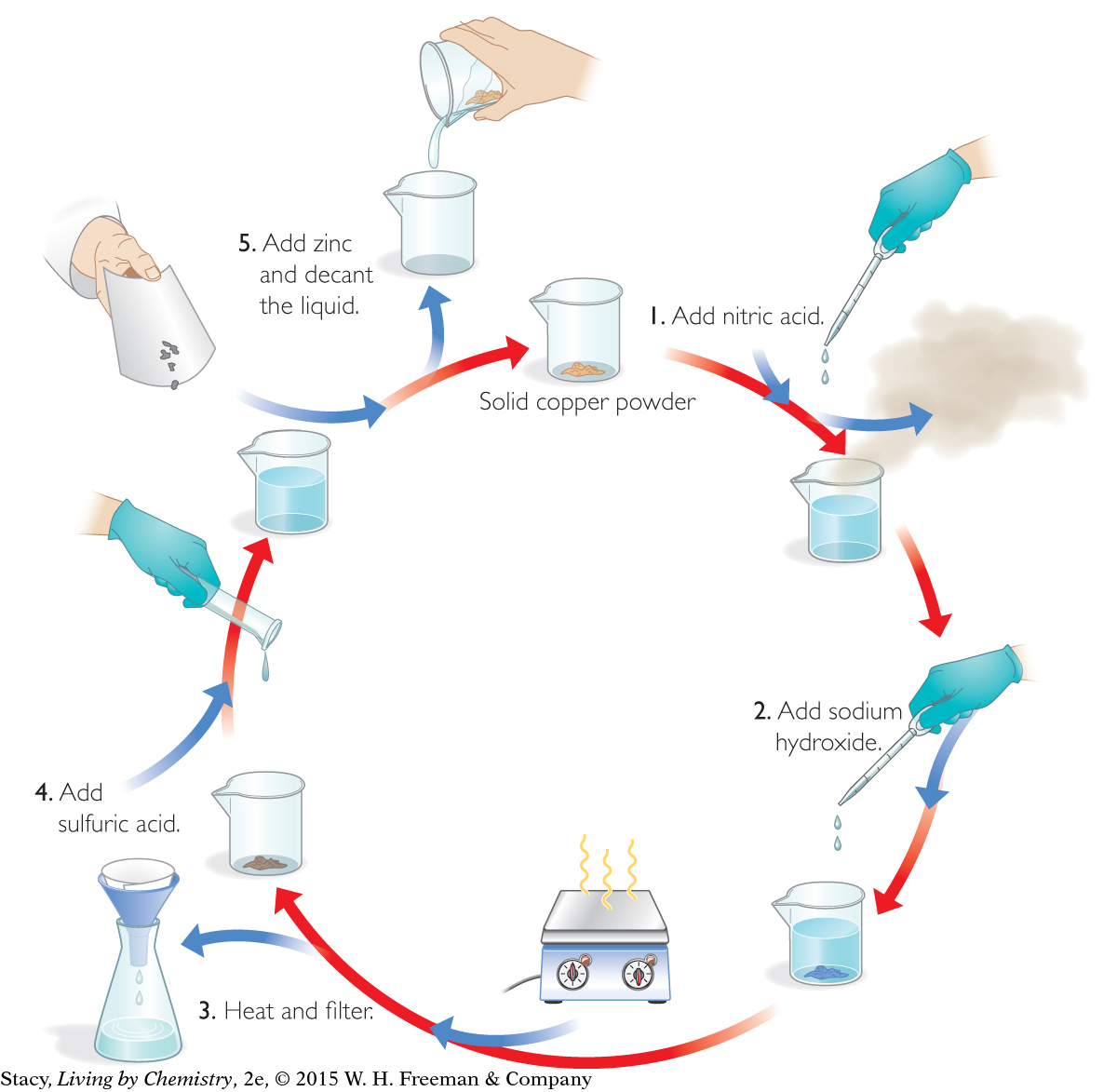
The Copper Cycle
28
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
Octopus blood is blue-green in color. It contains the copper-rich protein hemocyanin as opposed to the iron-rich hemoglobin found in vertebrates.

To make sense of the copper penny experiment, it is important to learn more about how copper can be transformed. Copper itself is an element, represented by the chemical symbol Cu(s). As a solid powder, copper has a distinctive orange-brown appearance.
In class, you transformed copper powder through a series of chemical reactions. If all worked well, copper should have reappeared at the end of the experiment. But what happened between the beginning and the end?
The diagram above shows the steps of the copper cycle. Start at the top and follow the arrows.
Evidence of Chemical Change
Evidence of Chemical Change
The five steps of the copper cycle are shown in these photos. In each step, a substance is changed into a different substance by mixing it with a liquid or by heating it. These changes are called chemical changes or chemical reactions. You can usually tell when a chemical reaction occurs because there is evidence of new substances forming. For instance, in class you observed some color changes when two of the liquids turned a bright blue color. You also observed the release of a brown gas and the formation of a dark-colored solid. These are all possible signs that new substances are forming. After a chemical reaction, you have new substances with properties that are different from the starting substances.

Copper powder
Ken Karp Photography
|

Step 1: After adding nitric acid
Ken Karp Photography
|

Step 2: After adding aqueous sodium hydroxide
Ken Karp Photography
|

Step 3: After heating
Ken Karp Photography
|

Step 4: After adding sulfuric acid
Ken Karp Photography
|

Step 5: After adding zinc
Ken Karp Photography
|
Interpreting Observations
Interpreting Observations
29
HISTORY CONNECTION
HISTORY
CONNECTION
Copper is not usually found as a pure element in nature. It is found in compounds. About 7000 years ago, people learned how to extract copper from rocks. They heated certain rocks together with charcoal in an oven, producing molten copper and carbon dioxide gas.

Look again at the first step in the copper-cycle diagram. When nitric acid was added to the copper, a blue liquid formed and a toxic brown gas left the beaker. But where did the copper powder go? You may have wondered if the copper left the beaker with the brown gas. However, copper reappeared at the end of the experiment. This is evidence that the copper doesn’t leave the beaker at any point in the cycle.
Was copper put back in again? If copper was not added in again and it did not leave with the brown gas, the only other explanation is that it never left the beaker at all. This would mean that the copper is present in copper-containing compounds at each step of the cycle. Copper must be present somewhere in the blue solutions and the blue solid that were observed. So, the copper was not created or destroyed as a result of its chemical journey.
Over many centuries, chemists have carried out procedures like the copper cycle. In doing so, they have learned about the properties of substances and even discovered new substances. They have also learned how to predict change. Chemists know that pouring nitric acid on copper produces a clear blue liquid and a brown gas. They have even determined the identity of the blue liquid and the brown gas. Perhaps further investigation will allow you to track the changes to the copper penny as well.
Example
Rusting Nail

An iron nail that stays in contact with water and air starts to form a reddish-brown coating called rust.
Is this a chemical change?
Is rust an element, a compound, or a mixture? Explain your thinking.
How could you gather more evidence to support your answer to part b?
Solution
Rust forms on the surface of the nail, so you can deduce that it is probably caused by exposure to water and air.
There is evidence of a new substance being formed on the surface of the nail. There is a color change and the rust is softer than iron. Therefore, a chemical change has taken place.
Rust is probably a compound, a combination of the iron from the nail plus one or more elements from the air or water.
To gather more evidence for chemical change, you could complete several experiments. For example, you could place a nail in pure water or in pure oxygen to test what the iron is reacting with. (Scientists have done this and found that iron rusts only in the presence of both oxygen and water. They have determined that rust is actually iron oxide hydroxide.)
LESSON SUMMARY
LESSON SUMMARY
30
KEY TERMS
chemical change
chemical reaction
What happens to matter when it is changed?
When one substance is transformed, or changed, into another substance, a chemical change has occurred. A chemical change is also called a chemical reaction. When chemical reactions occur, new substances with new properties are produced. Evidence of new substances, such as the production of a gas, a color change, or the formation of a solid in solution, is a sign that a chemical change has taken place. When substances undergo chemical changes, the elements themselves are not created or destroyed.
Exercises
Reading Questions
Explain what a chemical reaction is. What are some possible signs that a chemical reaction is taking place?
In your own words, explain how it might be possible to start with copper and end up with copper after a series of chemical reactions.
Reason and Apply
Look again at the copper-cycle diagram. After adding zinc, the blue solution turns colorless, and copper appears as a solid. What do you think happened to the zinc? Explain your thinking.
 Are cookies an example of a chemical change?Todd Wright/Blend Images/Thinkstock
Are cookies an example of a chemical change?Todd Wright/Blend Images/ThinkstockSuppose you use sugar, butter, eggs, and flour to make some cookie dough. You bake the dough in the oven until the cookies are done. Do the ingredients undergo a chemical change? Give evidence to support your answer.
Baking soda is a white powder used for baking or cleaning. When you mix baking soda, NaHCO3, with vinegar, C2H4O2, you get a clear colorless liquid and bubbles of CO2.

Specify the phase of each of the compounds.
Is this a chemical change? Give evidence.
Where is the sodium, Na, before the change? After the change?
Research how copper is extracted from a compound found in nature. Write a paragraph describing the process.