LESSON 9: Create a Table
37
THINK ABOUT IT
In the late 1860s, a Russian chemist and teacher named Dmitri Mendeleyev was looking for a way to organize the elements known at the time. He wanted to make it easier to remember and understand their chemical behavior. He started by organizing the elements into groups based on similarities in their properties. You can understand Mendeleyev’s organization of the elements by examining the information he used to sort them.
How is the periodic table organized?
To answer this question, you will explore
Properties of the Elements
A Table of Elements
Properties of the Elements
EXPLORING THE TOPIC
Properties of the Elements
Mendeleyev was intrigued by patterns in the properties of the elements. For example, tin, Sn, sodium, Na, and magnesium, Mg, are all shiny, silvery solids at room temperature.

Tin, Sn
© Nation Wong/Corbis
|

Sodium, Na
Martyn F. Chillmaid/Science Source
|

Magnesium, Mg
Andraz Cerar/iStockphoto
|
However, by itself, visual appearance is not a reliable characteristic for sorting the elements into groups. For instance, many elements are shiny, silvery solids at room temperature. And while both oxygen and neon are colorless gases, they are not similar enough in their other properties to be grouped together. Mendeleyev focused on three properties besides appearance to sort the elements: reactivity, the formulas of chemical compounds that form when the element combines chemically, and atomic mass.
REACTIVITY
Mendeleyev focused on the reactivity of elements to sort them. Reactivity is a property that describes how easily an element will combine with other substances to form new compounds. An element that is highly reactive combines rapidly with other substances. An explosion, smoke, or a flash of light is a sign that a reaction is proceeding quickly. For instance, when the metal sodium, Na, comes into the slightest contact with water, it reacts vigorously. Sodium even reacts with water vapor in the air. Magnesium, Mg, is another metal that reacts with water. Sodium and magnesium are both shiny, silvery metals that react with water. Based on their properties, these two metals might belong in the same group.
HISTORY CONNECTION
HISTORY
CONNECTION
It took thousands of years for human beings to discover the elements that are the building blocks of matter. Ancient civilizations discovered and used seven metals: gold (Au), copper (Cu), silver (Ag), lead (Pb), tin (Sn), iron (Fe), and mercury (Hg). These seven elements are called the metals of antiquity. Many of the early elements were discovered because they are less reactive than other elements and therefore more likely to be found in their pure forms.

38
FORMULAS OF COMPOUNDS
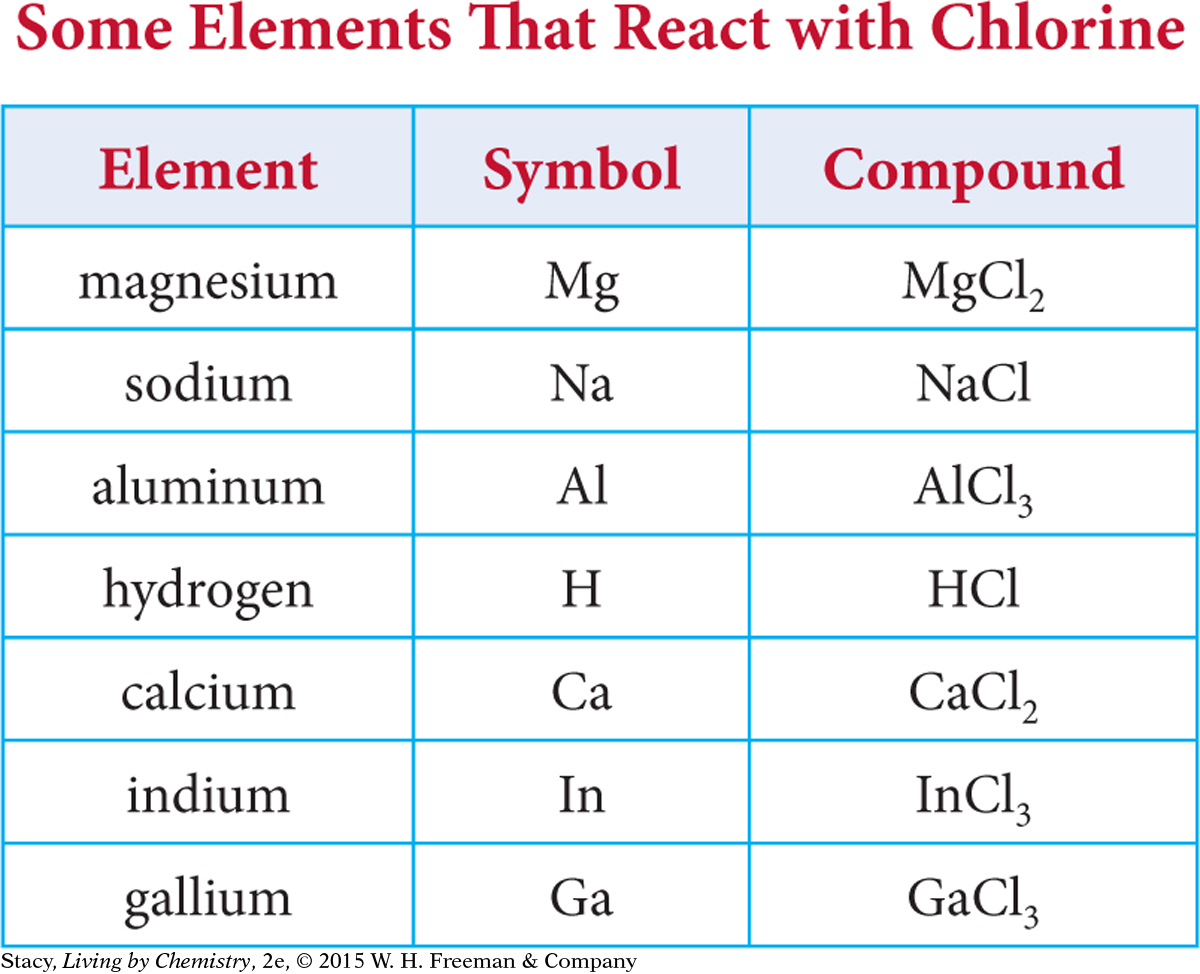
Mendeleyev also paid attention to which elements combine with which, and he noted the ratios in which their atoms combine. For example, magnesium, Mg, can combine with chlorine, Cl. When it does, it forms the compound magnesium chloride, MgCl2. This chemical formula indicates that atoms of magnesium and chlorine combine in a 1:2 ratio.
Sodium, Na, also reacts with chlorine, but it combines in a 1:1 ratio, forming sodium chloride, NaCl. Perhaps magnesium and sodium do not belong in the same group after all.
Examine the table, which shows some of the elements that react with chlorine and the compounds that are formed.
You can sort the elements into three groups according to the formulas of compounds with chlorine. In the Activity: Create a Table, you sorted these groups into separate columns, as Mendeleyev did.
ATOMIC MASS
Mendeleyev used another property, called atomic mass, to sort the elements. Each element is made of a different kind of atom. (You will study atoms in Lesson 11: Atomic Pudding.) All atoms of the same element have approximately the same mass. The mass of an atom is called its atomic mass and is measured in atomic mass units, or amu.
Atomic mass will be explained in more detail later in the unit. For now, simply keep in mind that each element has an average atomic mass that is expressed as a decimal number.
You can place the elements in order of their atomic masses. However, sorting the cards just by atomic mass doesn’t tell you which elements are similar in their properties.
A Table of Elements
A Table of Elements
39
Mendeleyev combined all of these sorting tactics to create his table. He put the elements with similar reactivity and chemical formulas of compounds into columns. Mendeleyev also sorted the elements in order of their atomic masses. He placed the lighter elements at the top of the columns and the heavier elements at the bottom. When he placed the columns next to each other, the atomic masses increased from left to right as well as from top to bottom.
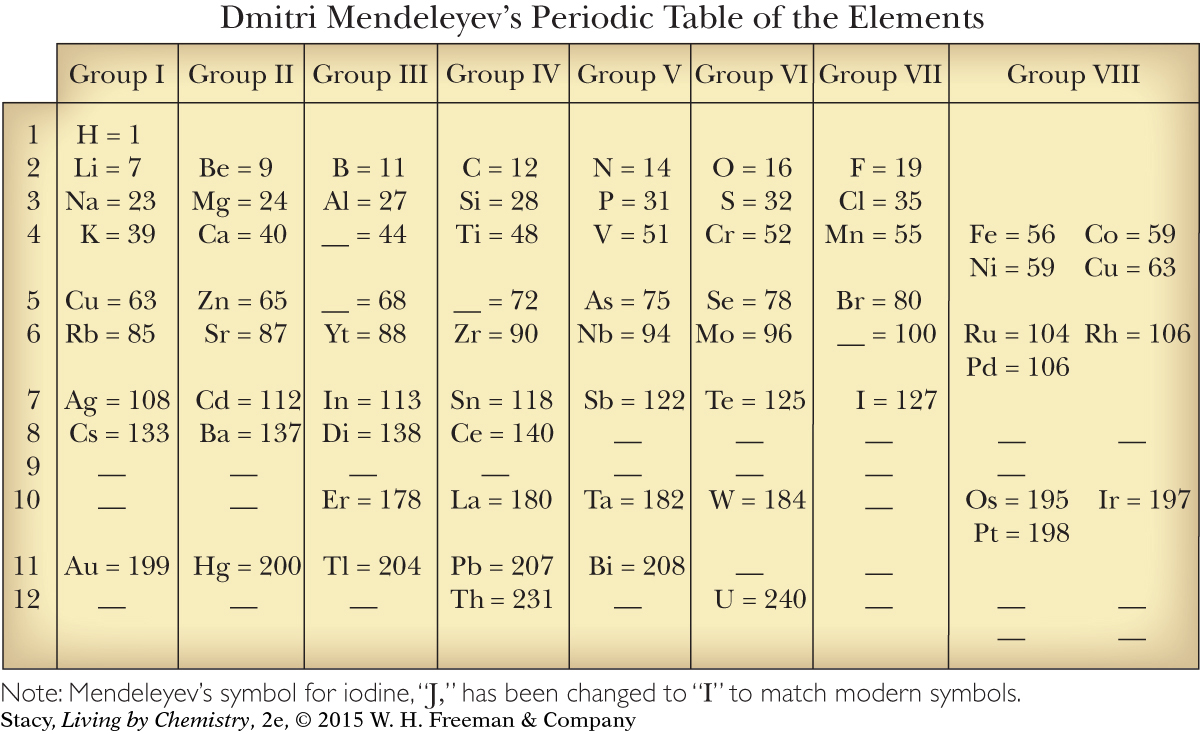
Mendeleyev organized the 63 elements that were known at the time into a table. In his table, the average atomic masses of the elements increase as you proceed across each row and down each column. The elements in each column have similar physical properties and reactivity, and they tend to form compounds with other elements in the same ratios. The table Mendeleyev created, with elements organized into rows and columns, became known as the periodic table of the elements.
The periodic table is an extremely useful organization of the elements. Mendeleyev was even able to predict the existence and properties of as-yet undiscovered elements based on gaps he located in his table.
Big Idea
Big Idea
Elements are arranged on the periodic table based on similarities in their chemical and physical properties.
LESSON SUMMARY
LESSON SUMMARY
How is the periodic table organized?
KEY TERMS
reactivity
average atomic mass
atomic mass unit, amu
periodic table of the elements
Dmitri Mendeleyev created one of the first organized tables of the elements. He sorted the elements based on their properties, specifically reactivity, the formulas of compounds created when elements chemically combine, and atomic mass. He organized the elements into a table so that their atomic masses increased in order across a row from left to right and down a column. The elements in each column of the table have similar properties. The table Mendeleyev created came to be called the periodic table of the elements.
40
Exercises
Reading Questions
List three properties of the elements that are useful in sorting the elements.
Do you expect carbon, C, to be more similar to nitrogen, N, oxygen, O, or silicon, Si? Why?
Reason and Apply
Use a reference book or the Internet to look up the average atomic masses and properties of silicon, Si, germanium, Ge, tin, Sn, phosphorus, P, antimony, Sb, sulfur, S, and selenium, Se.
Organize these elements into rows and columns.
List two properties that the elements in each column have in common.
Use a reference book or the Internet to look up some of the properties of iron, barium, and phosphorus. Explain why nails are made of iron but they are never made of barium or phosphorus. Suppose you have equal amounts of calcium, Ca, in two beakers. In one beaker, you react the calcium with oxygen, O. In the other beaker, you react the calcium with sulfur, S. The reaction with oxygen forms the compound calcium oxide, CaO.
What do you predict is the chemical formula of the compound formed from the reaction between calcium and sulfur?
Which compound has more mass, the compound containing calcium and oxygen, or the compound containing calcium and sulfur? Explain your thinking.
Use the Internet to research Dmitri Mendeleyev, the Russian chemist credited with the discovery of the periodic table of the elements. Write a brief paragraph describing Mendeleyev’s life and work. Include how he became a chemistry professor and how he came up with the idea for the periodic table. Find at least two different versions of the periodic table and bring a copy of each to class.
Write down what you think makes these two versions similar to each other.
Write down what you think makes these two versions different from each other.