Chapter 2 Summary
49

CHAPTER 2
Basic Building Materials
SUMMARY
KEY TERMS
element
chemical symbol
compound
chemical formula
phase
aqueous
chemical change (chemical reaction)
law of conservation of mass
reactivity
atomic mass
atomic mass units, amu
periodic table of the elements
atomic number
group
alkali metal
alkaline earth metal
halogen
noble gas
period
main group elements
transition elements
lanthanides
actinides
metal
nonmetal
metalloid
average atomic mass
Alchemy Update
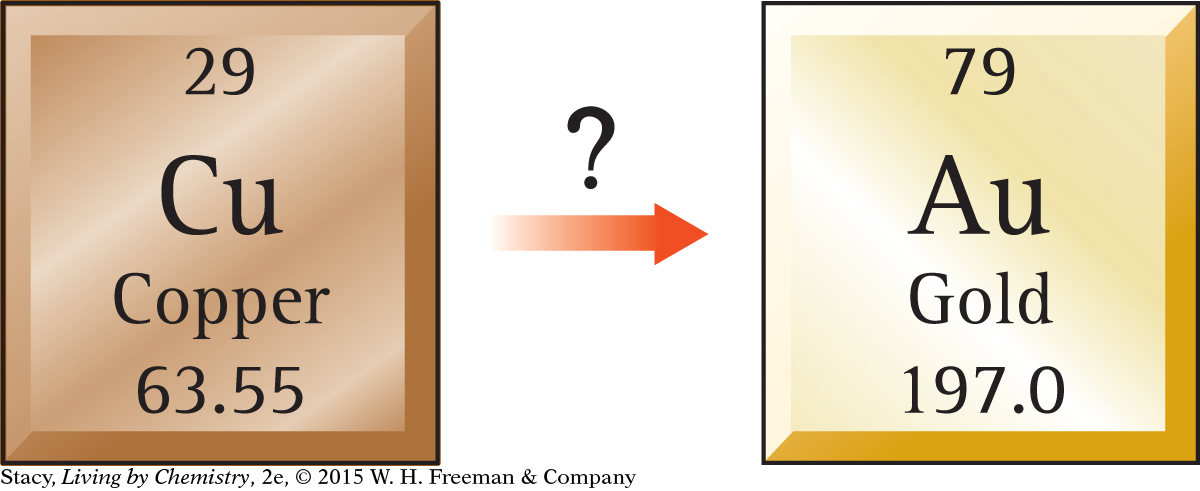
Can an element, such as copper, be transformed into gold through chemical processes? Copper and gold are in the same group on the periodic table and have similar properties. However, they are distinct elements with many differences, such as their appearances. While elements can react to form new compounds, from what you have learned so far it does not appear possible to change one element into another element.
REVIEW EXERCISES
Question 2.1
1. Make a list of all the information you can extract from the periodic table for the element gold.
Question 2.2
2. Explain the law of conservation of mass and how it relates to the copper cycle.
Question 2.3
3. What is a chemical formula, and what does it tell you?
Question 2.4
4. A filament for a light bulb must conduct electricity. Which of the elements listed below might be useful as a light bulb filament? Explain your thinking.
tungsten, W
sulfur, S
bromine, Br