LESSON 12: Atoms By Numbers: Atomic Number and Atomic Mass
56
THINK ABOUT IT
The element copper is made up of copper atoms. Likewise, gold is made up of gold atoms. We know that copper and gold are different elements. But what makes a copper atom different from a gold atom?
How are the atoms of one element different from those of another element?
To answer this question, you will explore
Atomic Number
Atomic Mass
The Periodic Table and Atomic Models
Atomic Number
EXPLORING THE TOPIC
Atomic Number
In Lesson 9: Create a Table, you learned how Mendeleyev arranged the elements in order of increasing atomic mass. Around 1913, Henry Moseley, a British scientist, discovered an amazingly simple and important property of the elements as well. He determined that the atoms of each element differ by one proton from the atoms of the element before it on the periodic table. The first element, hydrogen, H, has one proton, and the second element, helium, He, has two protons. The third element, lithium, Li, has three protons, beryllium, Be, has four protons, and so on.
The atomic number is equal to the number of protons in the nucleus of an element. The elements on the periodic table are arranged in order by their atomic numbers. So the element iron, Fe, with atomic number 26, has 26 protons in its nucleus.
Big Idea
Big Idea
If you know the number of protons in an atom, you know its atomic number and what element it is.
For a neutral atom, the atomic number is also equal to the number of electrons. This is because the overall charge of a neutral atom is 0. Protons have a +1 charge and electrons have a –1 charge. If the overall charge on an atom is 0, then the number of protons must be equal to the number of electrons.
Atomic Mass
Atomic Mass
The atomic mass is the mass of a single atom. The protons and neutrons account for almost all of the mass of an atom. Electrons have a much tinier mass by comparison. The atomic mass is approximately equal to the total mass of the neutrons and protons because electrons have so little mass.
CONSUMER CONNECTION
CONSUMER
CONNECTION
There are about 29,400,000,000,000,000,000,000 atoms of copper in each penny, or 29,400 quintillion atoms.

57
Every proton in every atom, whether it is an atom of gold or an atom of oxygen, has the same mass. Scientists assign a value of one atomic mass unit, 1 amu, to the mass of a single proton. Neutrons have almost exactly the same mass as protons, so each neutron also has a mass of 1 amu. To determine the mass of a single atom, you add the number of protons and neutrons.
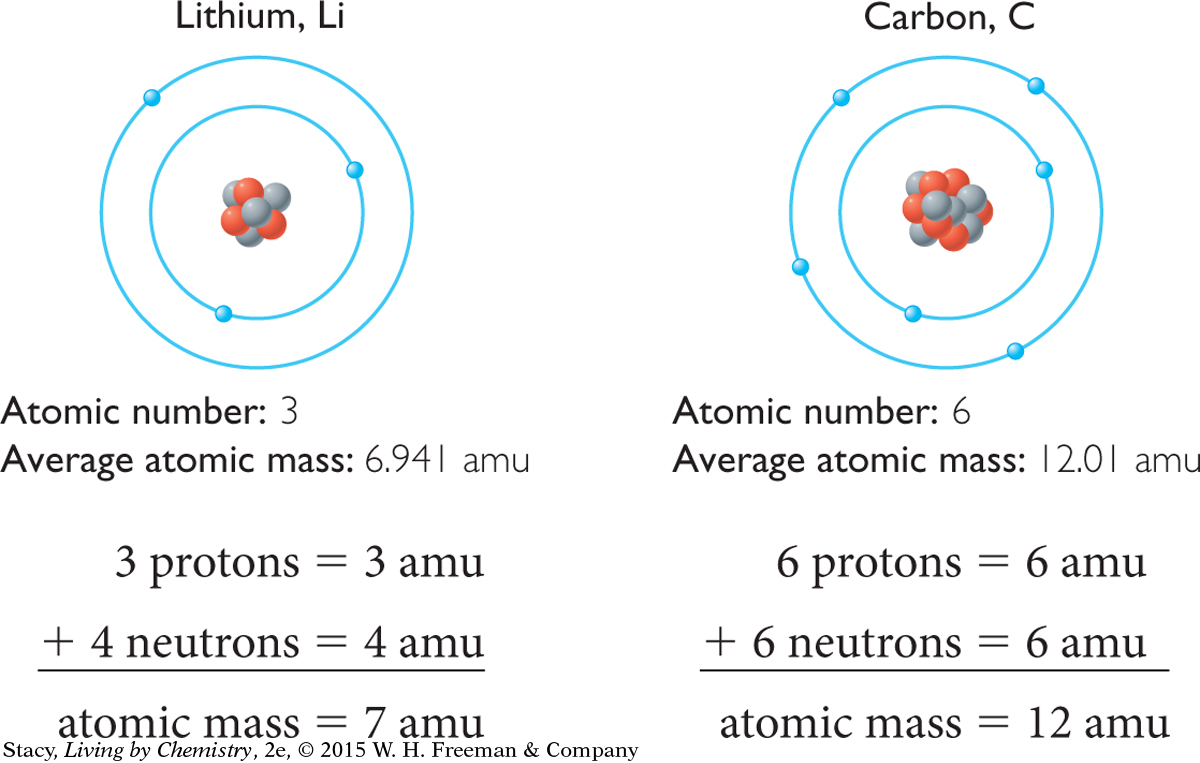
HOW MANY NEUTRONS?
If you look at the atomic models, you will notice that the numbers of protons and electrons are exactly the same as the atomic number, but the number of neutrons is sometimes different from the number of protons. So, how can the periodic table tell you how many neutrons are in an atom? If you know the mass of an atom and you know how many protons it has, you can find out how many neutrons it has by subtracting the number of protons from the atomic mass.
AVERAGE ATOMIC MASS
It turns out that not every atom of an element is identical. So, the decimal number in each element square of the periodic table is the average atomic mass of that element in atomic mass units, amu. This number can also be used to estimate the number of neutrons in a nucleus. Simply round the average atomic mass to the nearest whole number and subtract the atomic number (number of protons). For example, lithium has an average atomic mass of 6.941 amu, so a typical lithium atom probably has a mass of 7 amu. The atomic number of lithium is 3, so there are 3 protons. This accounts for 3 amu of the mass. The other 4 amu must be due to 4 neutrons.
In Lesson 13: Subatomic Heavyweights, you will investigate how the average atomic mass is arrived at and how atoms of an element may differ from one another.
Important to Know
When considering atomic mass, it is necessary to know whether you are focusing on the mass of one particular atom or the average mass of a group of atoms.
The Periodic Table and Atomic Models
The Periodic Table and Atomic Models
58
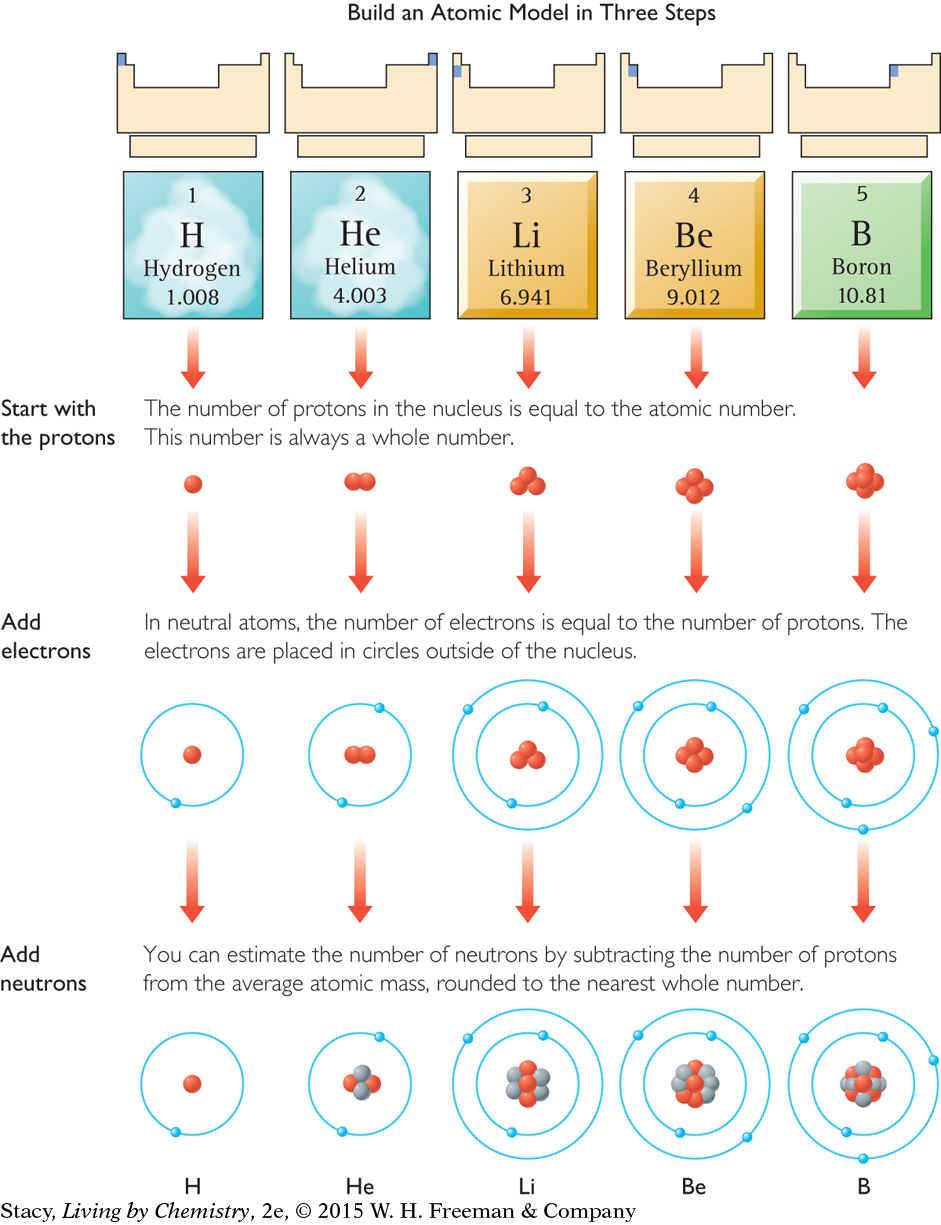
To draw an atomic model of a specific element, you must know the numbers of protons, neutrons, and electrons. You find this information on the periodic table. This illustration shows you how to get information about atomic structure from each square of the periodic table to build a basic atomic model of an element.
HISTORY CONNECTION
HISTORY
CONNECTION
Ernest Rutherford, a New Zealand─born scientist, is generally credited with the discovery of the proton. He was awarded the Nobel Prize in Chemistry in 1908, and his image appears on the New Zealand 100 dollar note.

59
Example
Copper and Gold Atoms
How is an atom of gold, Au, different from an atom of copper, Cu?
Solution
You can find the information you need on the periodic table. The atomic number of copper is 29. So neutral copper atoms have 29 protons and 29 electrons.
To estimate the number of neutrons in the atom, round the atomic mass to 64 and subtract the atomic number.
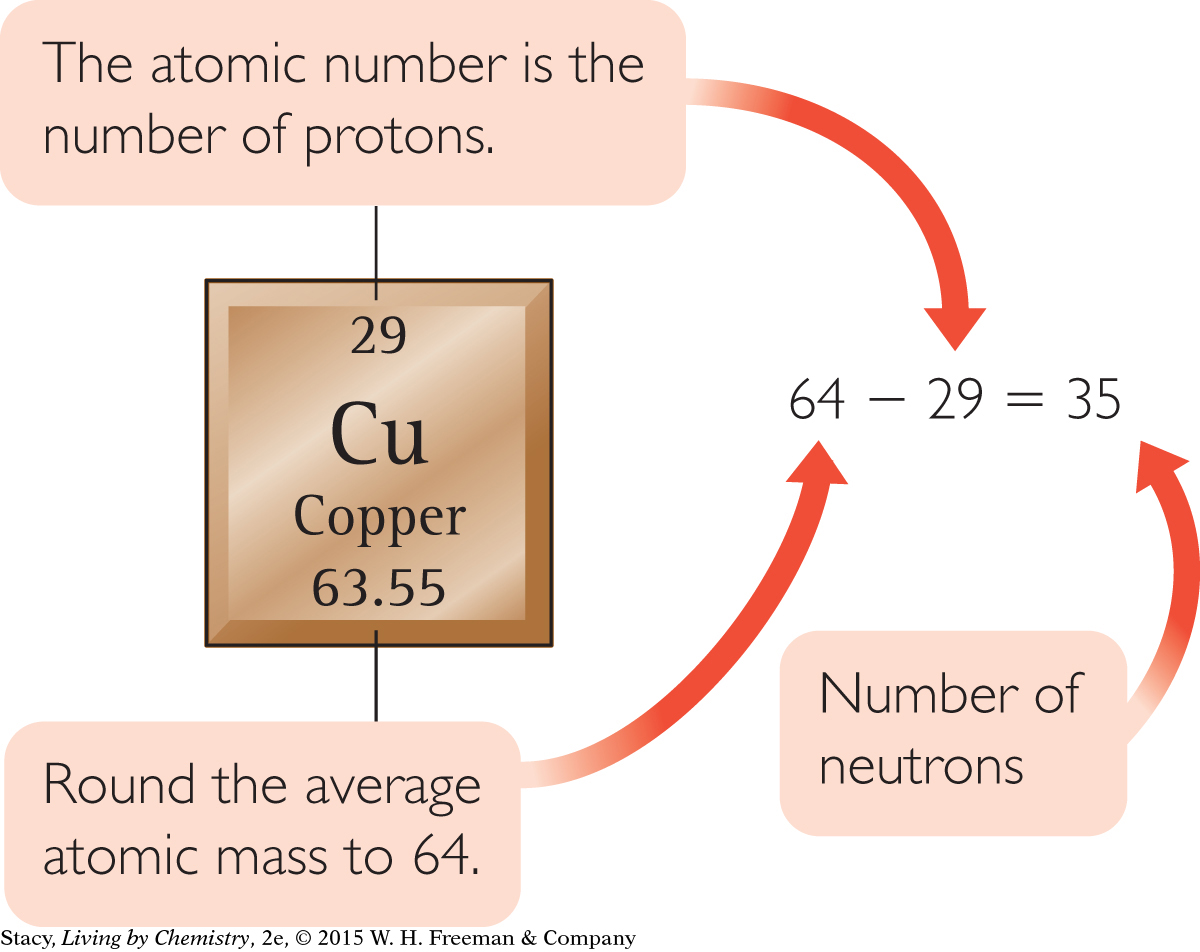
Number of protons = 29
Number of electrons = 29
Number of neutrons ≈ 35
You can follow the same steps for gold atoms.
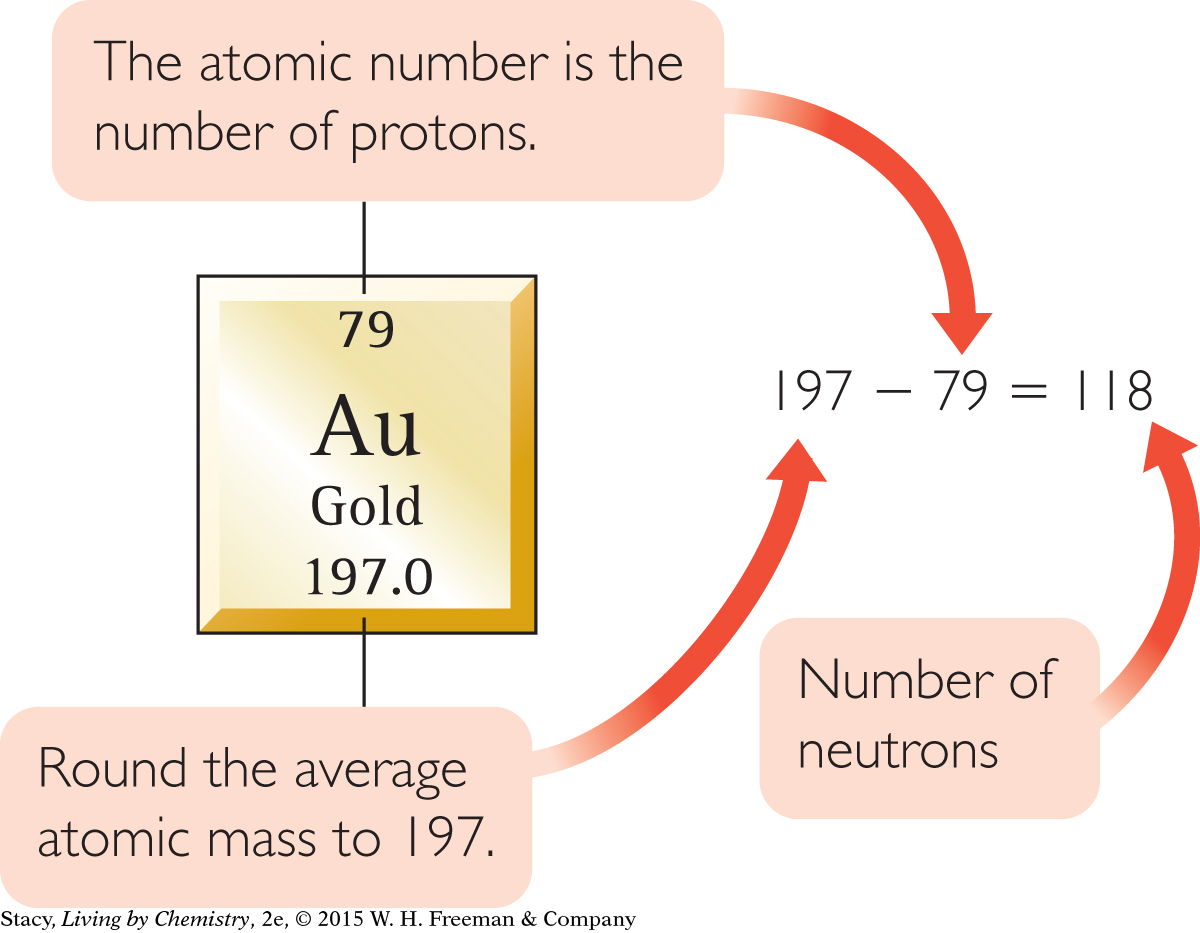
Number of protons = 79
Number of electrons = 79
Number of neutrons ≈ 118
So a gold atom has 50 more protons, 50 more electrons, and about 83 more neutrons than a copper atom.
LESSON SUMMARY
LESSON SUMMARY
60
How are the atoms of one element different from those of another element?
KEY TERM
atomic number
The periodic table reveals information about atomic structure. The atomic number of an element is equal to the number of protons in each of its atoms. The atomic number is also equal to the number of electrons in a neutral atom of an element. You can identify an element by the number of protons in the nucleus of an atom of the element. The protons and neutrons account for almost all the mass of an atom. Therefore, the mass of an atom in amu is approximately equal to the number of protons plus the number of neutrons. You can estimate the number of neutrons in an atom by subtracting the number of protons from the average atomic mass.
Exercises
Reading Questions
What does the atomic number tell you?
What does the atomic mass tell you?
Reason and Apply
If you have a sample of atoms and each atom has 12 protons in its nucleus, which element do you have?
If you want to identify an element, what one piece of information would you ask for? Explain your thinking.
Why does carbon, C, have a larger atomic mass than boron, B, even though they each have six neutrons?
Make a table like the one below. Use a periodic table to fill in the missing information.
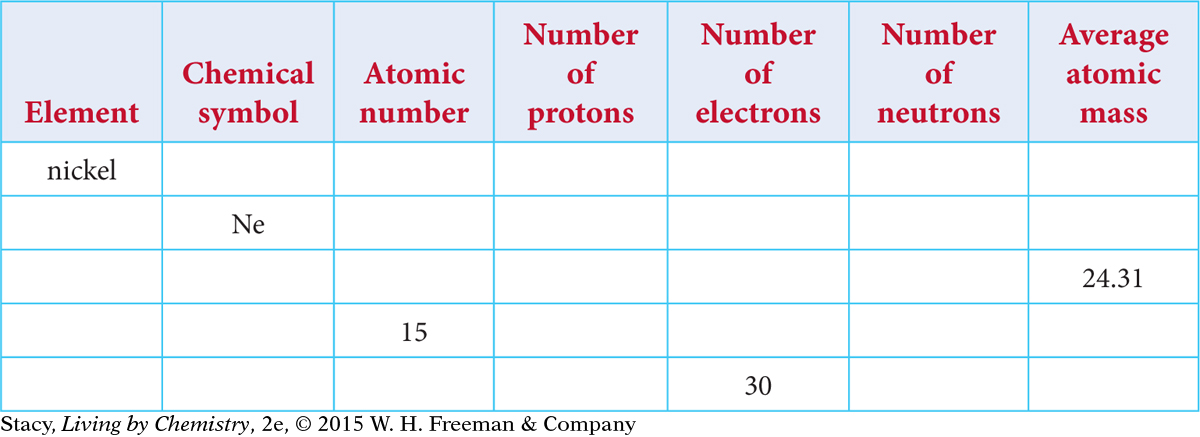
Draw a simple atomic model for an atom of neon, Ne.
Place the following elements in order from lowest number of protons to highest number of protons: S, Mg, N, Na, Se, Sr. Then give the following information about a neutral atom of each: name, atomic number, number of protons, number of electrons, group number.