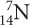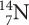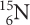LESSON 13: Subatomic Heavyweights: Isotopes
61
THINK ABOUT IT
It might surprise you that all atoms of the element copper are not exact replicas of one another. Although all copper atoms have many similarities, their masses can differ by 1 or 2 amu. What is the same and what is different about these atoms?
How can atoms of the same element be different?
To answer this question, you will explore
Isotopes
Average Atomic Mass
Isotopes
EXPLORING THE TOPIC
Isotopes
VARIATIONS IN THE NUCLEUS
Carbon is the sixth element on the periodic table. Its average atomic mass is listed as 12.01 amu. Why is the atomic mass listed as 12.01 rather than simply as 12?
Most of the carbon atoms that are found in nature do have a mass of 12 amu. However, for every 100 carbon atoms, it is typical to find one carbon atom with a mass of 13 amu and even more rarely a carbon atom with a mass of 14 amu. This makes the average atomic mass 12.01 amu. Consider the structures of these three varieties of carbon atom. A model of each is shown here.
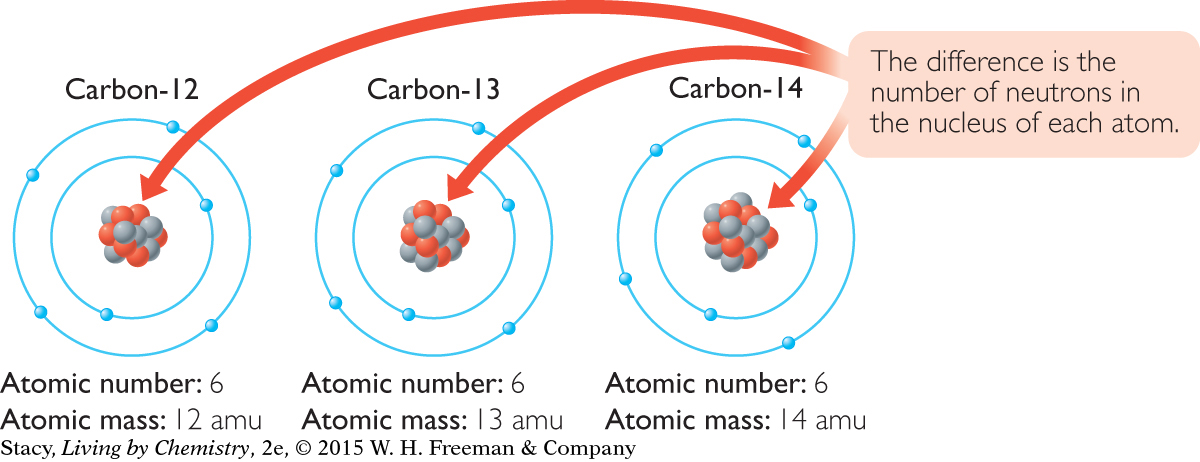
Compare the nucleus of each atom. Each has 6 protons. That is what makes them all carbon atoms. Recall that each atom of an element has the same number of protons as every other atom of that element.
However, one carbon atom has 6 neutrons, another carbon atom has 7 neutrons, and the third has 8 neutrons. The mass of an atom is the sum of the number of protons and neutrons, so one carbon atom has a mass of 12 amu and the others have masses of 13 and 14 amu. These atoms are referred to as carbon-12, carbon-13, and carbon-14 to show that they are all atoms of carbon but have different masses.
62
TECHNOLOGY CONNECTION
TECHNOLOGY
CONNECTION
A mass spectrometer is used to determine the isotopic composition of an element. It makes an extremely accurate measurement of the mass of an individual atom, using the principle that a heavier particle will travel a straighter path through a magnetic field than a lighter particle.
Atoms of an element that have different numbers of neutrons are called isotopes. Carbon has three isotopes. In nature, almost all the elements have at least two isotopes. A few elements have as many as ten isotopes.
ISOTOPE SYMBOLS
Symbols for the three isotopes of carbon are shown below. Notice that the mass of each isotope is shown as a superscript number (on top). This number is a whole number. It is the sum of the numbers of protons and neutrons in the atom and is sometimes called the mass number. The subscript number (on the bottom) is the atomic number of the element, in this case, 6. The number of neutrons in each atom is equal to the top number minus the bottom number.
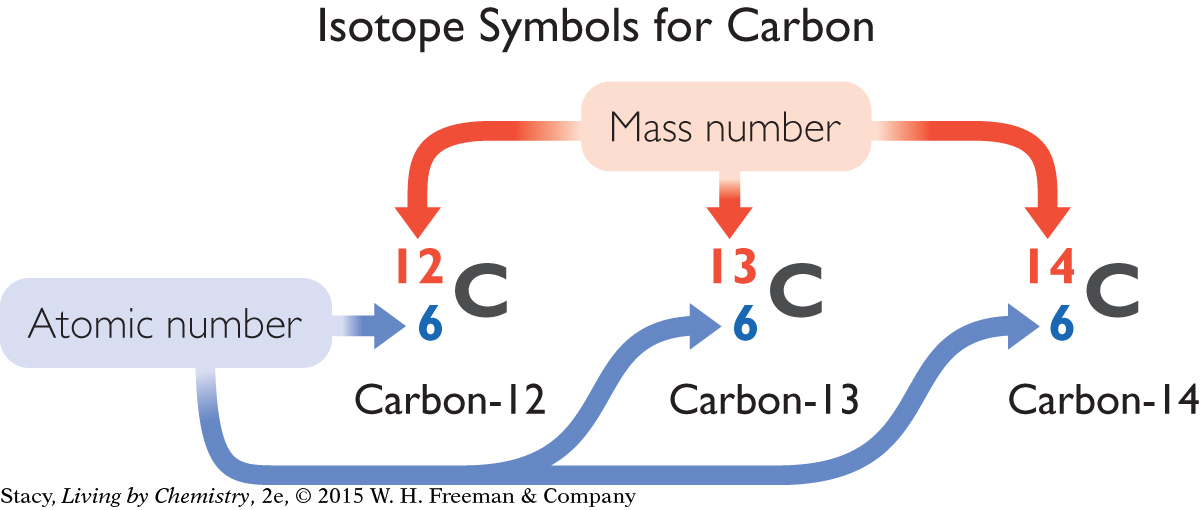
These three isotopes are nearly identical in their properties. For example, they all form the same compounds.
Big Idea
Big Idea
Isotopes are atoms of the same element but with different numbers of neutrons.
Average Atomic Mass
Average Atomic Mass
You may have noticed that the average atomic mass values in each square of the periodic table are decimal numbers, usually to the nearest hundredth of a unit. These values are averages of the masses of the isotopes in a sample. For example, neon has an average atomic mass of 20.18 amu. How is this average calculated?
63
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
Less common isotopes are more easily detectable, so researchers often use them to trace how an element moves through a living creature or the environment. For example, using a fertilizer that is enriched in nitrogen-15 allows a scientist to track the nitrogen atoms to see how much nitrogen a plant is using.

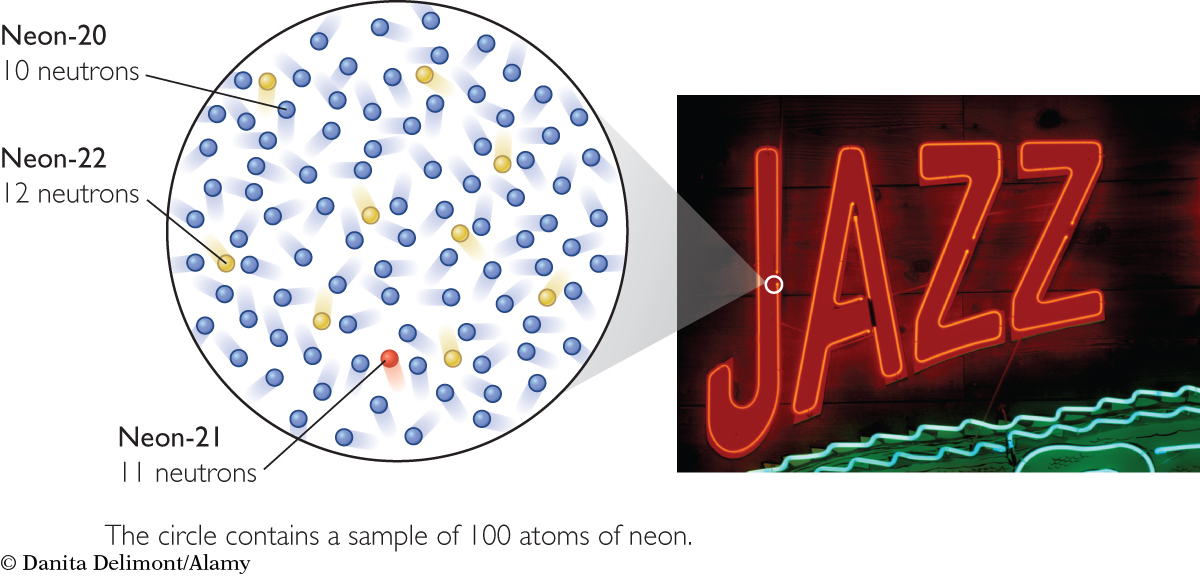
About 90% of all neon atoms have an atomic mass of 20 amu, 9% have an atomic mass of 22 amu, and 1% have an atomic mass of 21 amu. By considering a random sample of 100 neon atoms, you can calculate their average atomic mass like this:
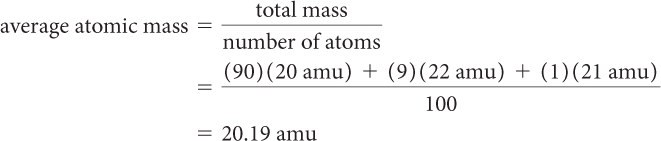
As you can see, this number is nearly identical to 20.18, the average atomic mass listed for neon on the periodic table.
The percentage of each isotope of an element that occurs in nature is called the natural abundance of the isotope. For example, the natural abundance of neon-20 is about 90.48%.
Important to Know
The mass of an isotope refers to the mass of a single specific atom of an element. The average atomic mass given on the periodic table is the average of the masses of all the isotopes in a large sample of that element.
Example
Isotopes of Copper
There are two different isotopes of copper. The isotope names and symbols are given here.
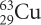
|
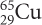
|
| copper-63 | copper-65 |
Explain why both symbols have 29 as the bottom number.
Explain how the two isotopes are different from each other.
Scientists have found the natural abundances of each isotope: 69% copper-63 and 31% copper-65. Explain why the average atomic mass listed on the periodic table for copper is 63.55.
Solution
Copper’s atomic number is 29, so both isotope symbols have a subscript 29 indicating 29 protons.
The two isotopes have different atomic masses. Both isotopes have 29 protons, so copper-63 has 34 neutrons and copper-65 has 36 neutrons.
You could consider a sample of 100 atoms of copper and calculate their average mass. The average mass of the 100 atoms is determined by adding the masses of the 100 atoms and dividing this total by 100.
This is very close to the value found on the periodic table.
Otherwise, you can convert each isotope’s percent natural abundance to a decimal number, then multiply this by the isotope’s mass number. Do this for each isotope, then add the products:
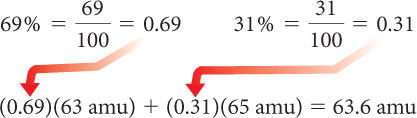
LESSON SUMMARY
LESSON SUMMARY
64
How can atoms of the same element be different?
KEY TERMS
isotope
mass number
average atomic mass
Elements are composed of nearly identical atoms, each with the same number of protons. However, not every atom of an element has the same number of neutrons in its nucleus. Atoms of an element with different numbers of neutrons are called isotopes. Because neutrons account for part of the mass of an atom, isotopes have different masses. The average atomic mass is an average of the masses of all the different isotopes, taking natural abundance into account.
Exercises
Reading Questions
Explain the differences between atomic number and atomic mass.
Explain the difference between the average atomic mass given on the periodic table and the mass of an atom.
Reason and Apply
How are potassium-39, potassium-40, and potassium-41 different from each other? Write the isotope symbols for the three isotopes of potassium.
How many protons, neutrons, and electrons are in each?
fluorine-23
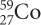
molybdenum-96
An isotope of iron, Fe, has 26 protons and 32 neutrons.
What is the approximate mass of this isotope?
How would you write the symbol for this isotope?
Find the element phosphorus, P, on the periodic table.
What is the average atomic mass of phosphorus?
What is its atomic number?
Predict which isotope you would find in greatest abundance for phosphorus.
Chlorine, Cl, is 76% chlorine-35 and 24% chlorine-37. Determine the average atomic mass of chlorine.
Lithium, Li, is 7.6% lithium-6 and 92.4% lithium-7. Determine the average atomic mass of lithium.
Which isotope of nitrogen is found in nature? Explain your reasoning.