LESSON 16: Old Gold: Formation of Elements
77
THINK ABOUT IT
The element gold is essentially a collection of identical atoms. Gold has only one stable isotope. So, every gold atom has 79 protons, 79 electrons, and 118 neutrons. Perhaps it is possible to make gold atoms by using nuclear processes to add or subtract protons, neutrons, and electrons. To understand if this is possible, it is useful to consider what processes lead to the creation of new elements.
How are new elements formed?
To answer this question, you will explore
Making New Elements
Writing Nuclear Equations
Nuclear Chain Reactions
Making New Elements
EXPLORING THE TOPIC
Making New Elements
Some nuclear reactions occur without intervention on Earth when radioactive isotopes decay. It is much more difficult to change the nucleus of a stable atom. However, there are places with the right conditions and enough available energy to change these nuclei. Nuclear changes take place continuously in the Sun and other stars. And on Earth, scientists can carry out certain nuclear reactions in specially designed facilities like nuclear reactors or particle accelerators.
New elements can be created through nuclear fission or nuclear fusion. Whether a nucleus is split apart or joined with another nucleus, both processes result in the formation of different elements. And both processes require an energetic push to get them started.
Big Idea
Big Idea
The only way to change one element into another is to change the number of protons in the nucleus.
ASTRONOMY CONNECTION
ASTRONOMY
CONNECTION
The Sun turns 600 million tons of hydrogen into helium every second! The energy released as a result of this nuclear fusion provides the brilliant sunlight we see every day and the intense heat that warms our planet, even from 93 million miles away. There is enough hydrogen in the Sun to keep the Sun burning for another 5 billion years.

MAKING ELEMENTS IN THE STARS
The creation of new elements through nuclear chemistry is called nucleosynthesis. Most nucleosynthesis takes place far from Earth, inside stars.
The Sun is a giant ball of hydrogen, H, and helium, He. The amazing light and heat energy that radiates from the Sun is the result of continuous fusion reactions. However, the Sun is not hot enough to produce elements beyond helium on the periodic table. Higher temperatures are needed for the formation of larger atoms.
78
Stars do not exist forever. Some stars collapse in on themselves after millions of years. Other stars explode and become what astronomers call supernovas. Where does gold come from? Small stars like our own can produce only helium. Larger, hotter stars can produce heavier elements up to iron. Only a supernova explosion can produce elements heavier than iron, including gold.
Writing Nuclear Equations
Writing Nuclear Equations
Nuclear reactions can be expressed as nuclear equations. You can use nuclear equations to track the number of neutrons and protons in the atoms, along with the identity of the products. Nuclear equations use isotope symbols to represent each particle. The alpha decay of an isotope of barium-140 to form xenon-136 can be written as a nuclear equation using α for the alpha particle:
An alpha particle is the same as a helium nucleus, so it can also be written this way:
In the second equation, you can track the protons and the mass that is removed from the barium nucleus. Notice that the equation is balanced numerically. The mass numbers on both sides are equal: 140 = 4 + 136. The numbers of protons are also equal on both sides: 56 = 2 + 54. In radioactive decay, the starting isotope is called the parent isotope and the resulting isotope is called the daughter isotope. In this equation, 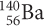 is the parent isotope and
is the parent isotope and 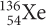 is the daughter isotope.
is the daughter isotope.
Beta decay can be shown in two ways as well, because a beta particle is the same as an electron and is sometimes shown as e–, or in isotope form as 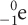 .
.
Recall that the beta particle, β, comes from a neutron in the nucleus splitting into a proton and an electron. The new element 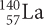 has a higher atomic number because it has one more proton in its nucleus than barium.
has a higher atomic number because it has one more proton in its nucleus than barium.
Fusion equations have two starting isotopes coming together to form a new product. When a carbon-12 isotope fuses with a nitrogen-14 isotope to form an isotope of aluminum, the equation looks like this:
Notice once again that the equation is balanced numerically on both sides.
Example
Radium-222
Write the equation representing the alpha decay of radium-222. What is the daughter isotope?
Solution
Find radium, Ra, on the periodic table. Its atomic number is 88. Now you can write the isotope symbol for radium, 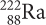 .
.
79
An alpha particle is the same as a helium nucleus. Because the alpha particle is emitted, it goes on the right side of the equation. Balancing the equation gives the resulting nucleus, which has atomic number 86, and which is radon, Rn.
The daughter isotope is radon-218.
Nuclear Chain Reactions
Nuclear Chain Reactions
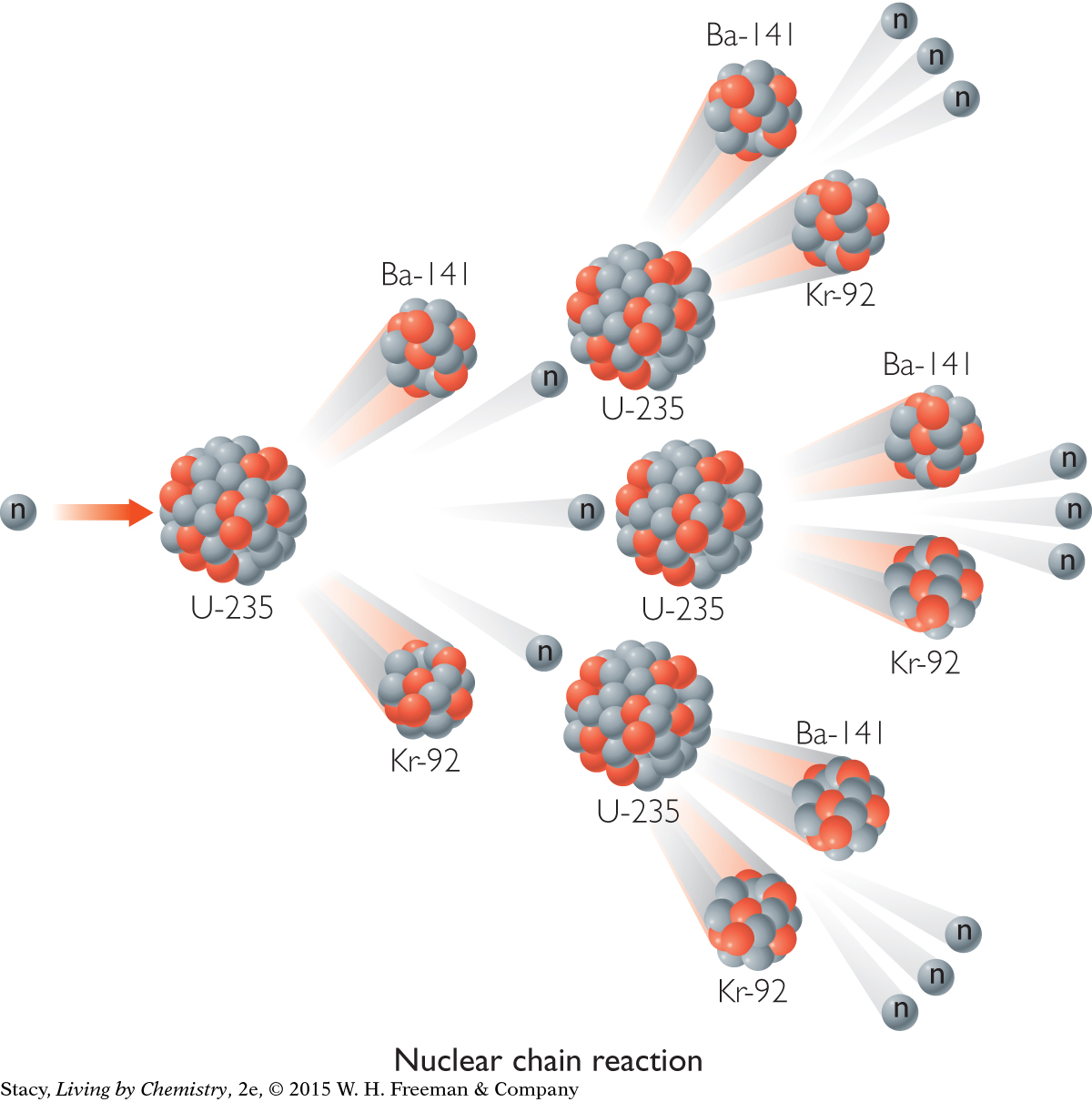
Nuclear fission can be provoked by striking nuclei with fast-moving particles, such as neutrons. And, sometimes, one nuclear reaction can lead to others. A nuclear chain reaction can take place when the neutrons emitted strike surrounding nuclei, causing them to split apart as well.
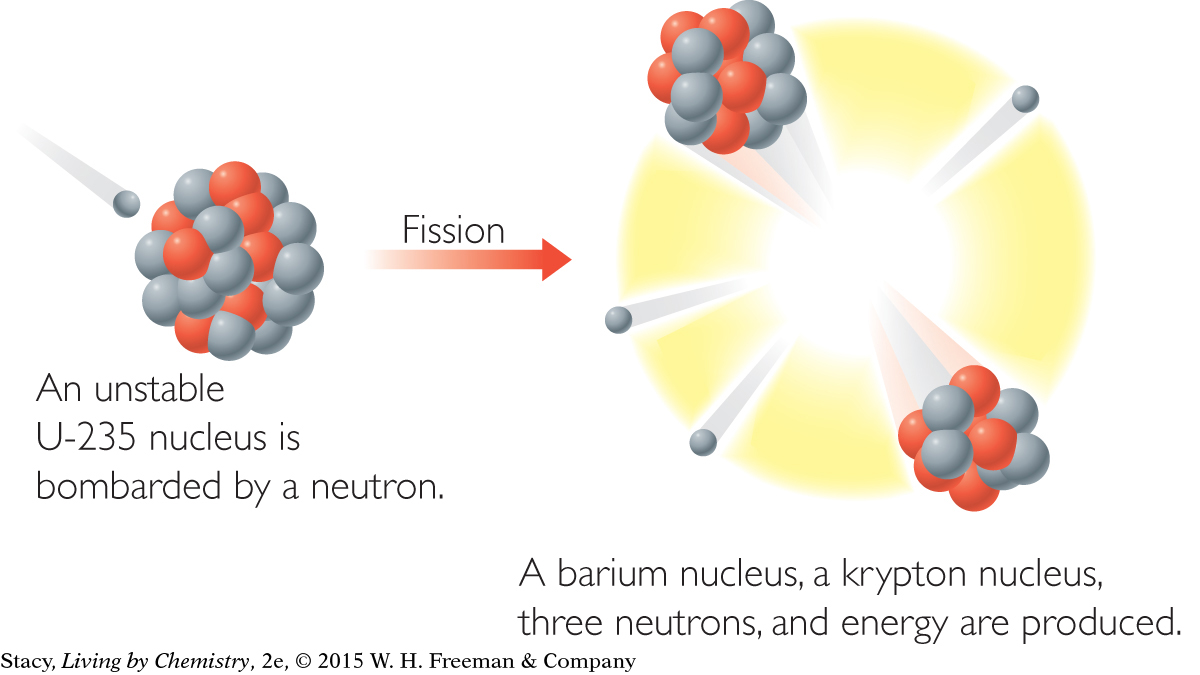
For example, natural uranium consists of three isotopes: uranium-238, uranium-235, and uranium-234. All of these uranium isotopes are mildly radioactive but are still fairly stable. Uranium isotopes can be provoked into undergoing fission reactions. Scientists do this by firing a neutron at a uranium atom. This results in the formation of two smaller isotopes and the emission of three other neutrons, which in turn cause other uranium isotopes to undergo fission. The result is a chain reaction releasing enormous amounts of energy.
80
Scientists have learned how to control and harness nuclear fission here on Earth. Nuclear fission is used to produce electricity, power submarines, and propel a variety of large ships. Uranium-235 is the isotope most commonly used for nuclear power.
If a nuclear chain reaction is controlled and the energy is released slowly, it can be used to generate electricity. If the energy is released all at once, the result is a nuclear explosion.
MAKING NEW GOLD
Most of the elements found on Earth are the products of fusion reactions in stars that exploded billions of years ago. The elements have been here for a very, very long time. There has been little change in the amounts of these elements. Small changes to the amounts of each element occur over time due to radioactive decay and fission. The gold we have on Earth came from supernova explosions that may have taken place billions of years ago. The prospect of making large quantities of new gold through nuclear chemistry appears slim.
LESSON SUMMARY
LESSON SUMMARY
How are new elements formed?
KEY TERMS
nuclear equation
parent isotope
daughter isotope
nuclear chain reaction
Elements are formed through nuclear reactions. Radioactive decay, nuclear fission, and nuclear fusion are all possible sources of new elements. The elements we have on Earth came from fusion reactions in stars and supernova explosions long ago. It is difficult to cause nuclear fusion to occur on Earth because large amounts of energy are required to fuse nuclei. Nuclear fission, on the other hand, has been harnessed as a source of power. The power that is generated by nuclear plants is a result of a controlled fission chain reaction.
Exercises
Reading Questions
Describe four processes that result in the formation of new elements.
What is a nuclear chain reaction?
Reason and Apply
Write the nuclear equation for the beta decay of cerium-141.
Write the nuclear equation for the alpha decay of platinum-191.
Write a nuclear equation for the formation of iron-54 through fusion.
Consider the fission of uranium-235.
The fission of uranium-235 begins with the addition of a neutron to the nucleus. What isotope is formed? Give the isotope symbol.
After the neutron is added, the uranium atom is more unstable and undergoes fission. A possible set of products is krypton-94, barium-139, and 3 neutrons. How many protons are in these products?
Were any protons lost?
Write a paragraph explaining how a nuclear reactor works. Be sure to explain the purpose of the control rods.