LESSON 24: Shell Game: Electron Configurations
116
THINK ABOUT IT
Recall that the chemistry of the elements is closely related to the number of valence electrons in their atoms. The valence electrons are found in the outermost electron shell of an atom.
What does the periodic table indicate about the arrangements of electrons?
To answer this question, you will explore
Subshells in Atoms
Electron Configurations
Connecting the Periodic Table to Electron Arrangements
Noble Gas Shorthand
Subshells in Atoms
EXPLORING THE TOPIC
Subshells in Atoms
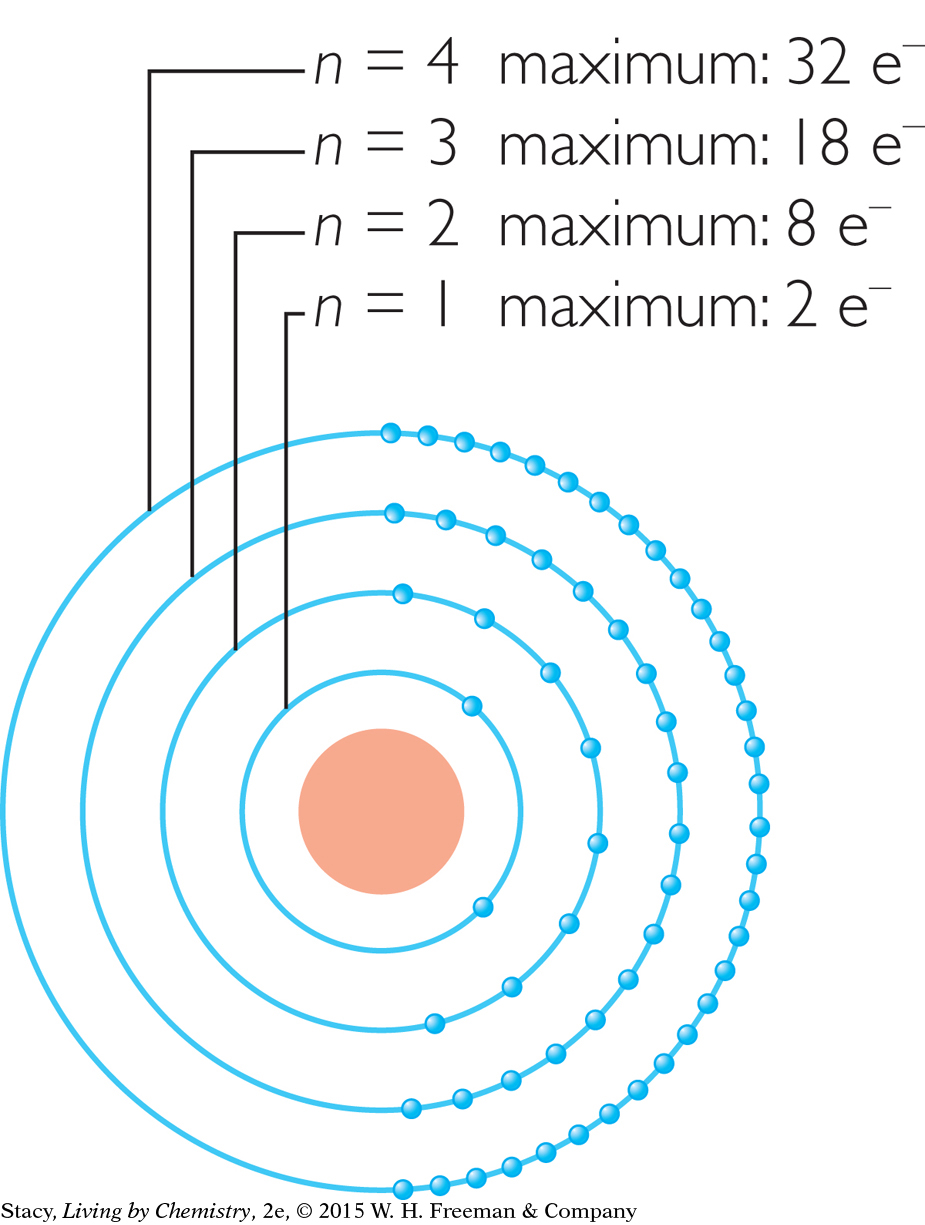
Electrons are arranged into shells numbered n = 1, 2, 3, and so on. The number of electron shells in an atom is the same as the number of the period where the element is located on the periodic table. Each shell has a maximum number of electrons. For instance, the n = 2 shell cannot have more than 8 electrons.
Scientific evidence has led chemists to propose that electron shells are further divided into electron subshells. Imagine magnifying the basic atomic model and finding that each shell is composed of subshells. Notice that the number of subshells that a shell has is equal to n.
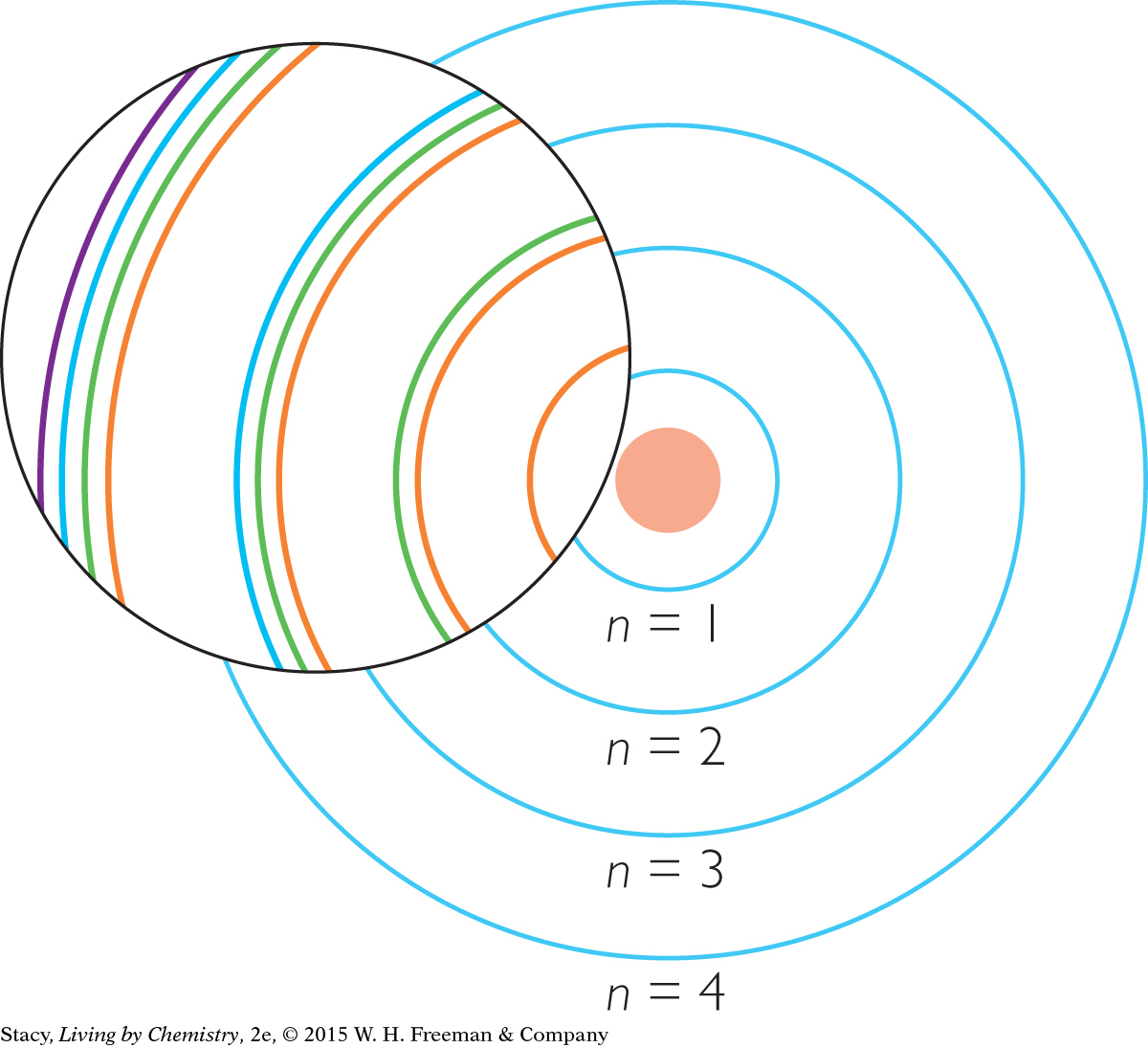
THE s, p, d, AND f SUBSHELLS
117
The subshells have special names. They are called the s, p, d, and f subshells. Just like the basic shells, each subshell has a maximum capacity of electrons. The s subshells can have a maximum of 2 electrons, p subshells can have a maximum of 6 electrons, and d subshells can have a maximum of 10 electrons. Finally, f subshells can have a maximum of 14 electrons.
Notice that the name of each subshell is labeled using both the basic shell number and the subshell letter (1s, 2s, 2p, and so on).
Example
Electron Arrangements
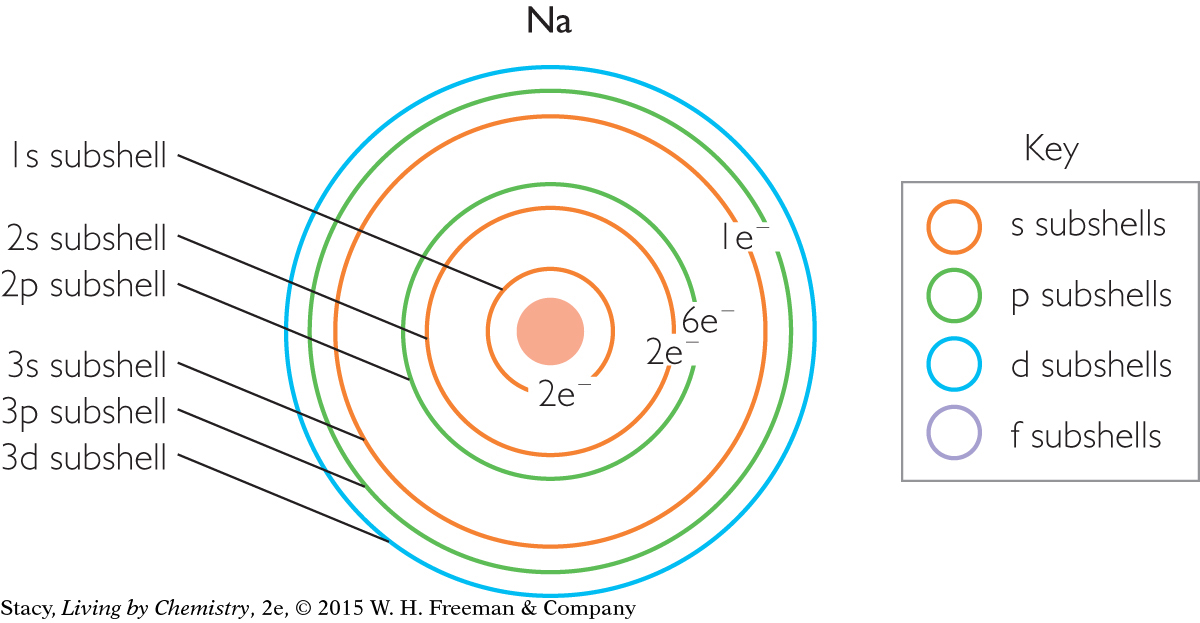
Use the illustration of the subshells in a sodium atom above to help you answer these questions:
How many total electrons are there in a sodium, Na, atom? Which shells are they in?
How many valence electrons does sodium have? Which subshell are they in?
How many electrons are there in the 3s subshell of sodium? In the 3p subshell?
Solution
The atomic number of sodium is 11.
There are a total of 11 electrons in a neutral sodium atom. The electrons are in shells, n = 1, n = 2, n = 3.
Sodium has one valence electron, located in the 3s subshell.
There is one electron in the 3s subshell of sodium, and none in the 3p subshell.
Electron Configurations
Electron Configurations
It can be time-consuming to draw subshell models of the atoms to show the arrangements of the electrons, especially for atoms with large atomic numbers. Chemists have developed a shorthand notation called an electron configuration to keep track of the electrons in an atom. The electron configurations for the first ten elements are shown here.
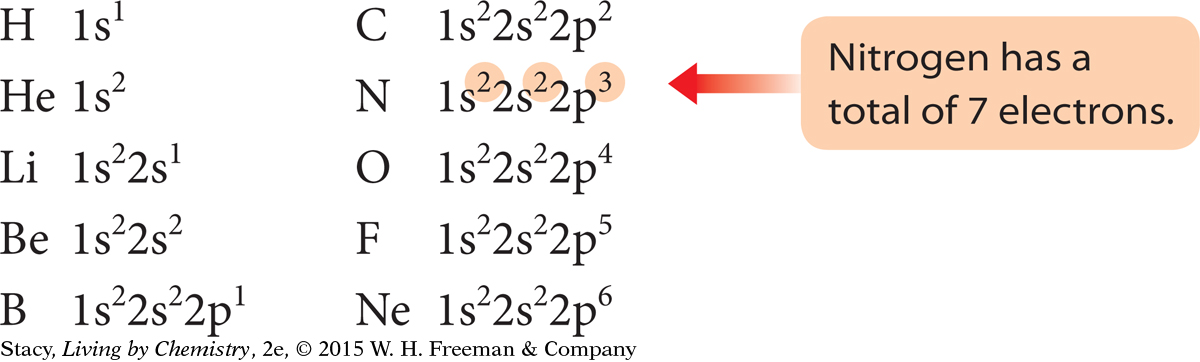
118
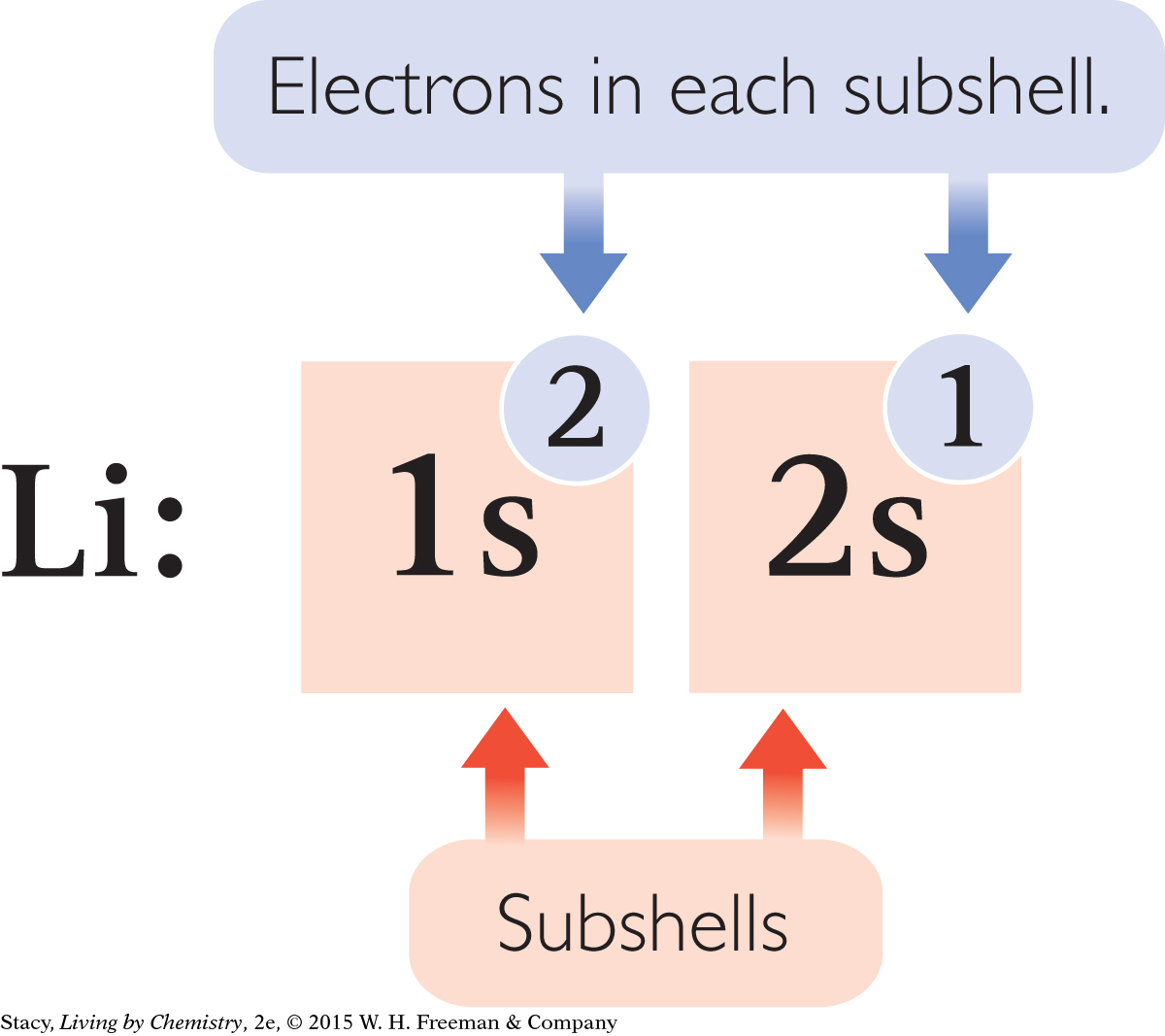
Each subshell is written using the shell number and the subshell letter. In addition, the number of electrons in each subshell is indicated with a superscript number.
Notice that the superscript numbers add up to the total number of electrons for that atom.
The sequence in which electrons fill up the subshells is 1s, 2s, 2p, 3s, 3p. After the element argon, the pattern changes slightly.
Example
Electron Configuration of Sulfur
Write the electron configuration for sulfur, S.
Solution
Sulfur is located in the third row in Group 6A. The atomic number of sulfur is 16, so there are 16 electrons that need to be distributed in subshells, beginning with the 1s subshell.
The electron configuration of sulfur is 1s22s22p63s23p4.
Connecting the Periodic Table to Electron Arrangements
Connecting the Periodic Table to Electron Arrangements
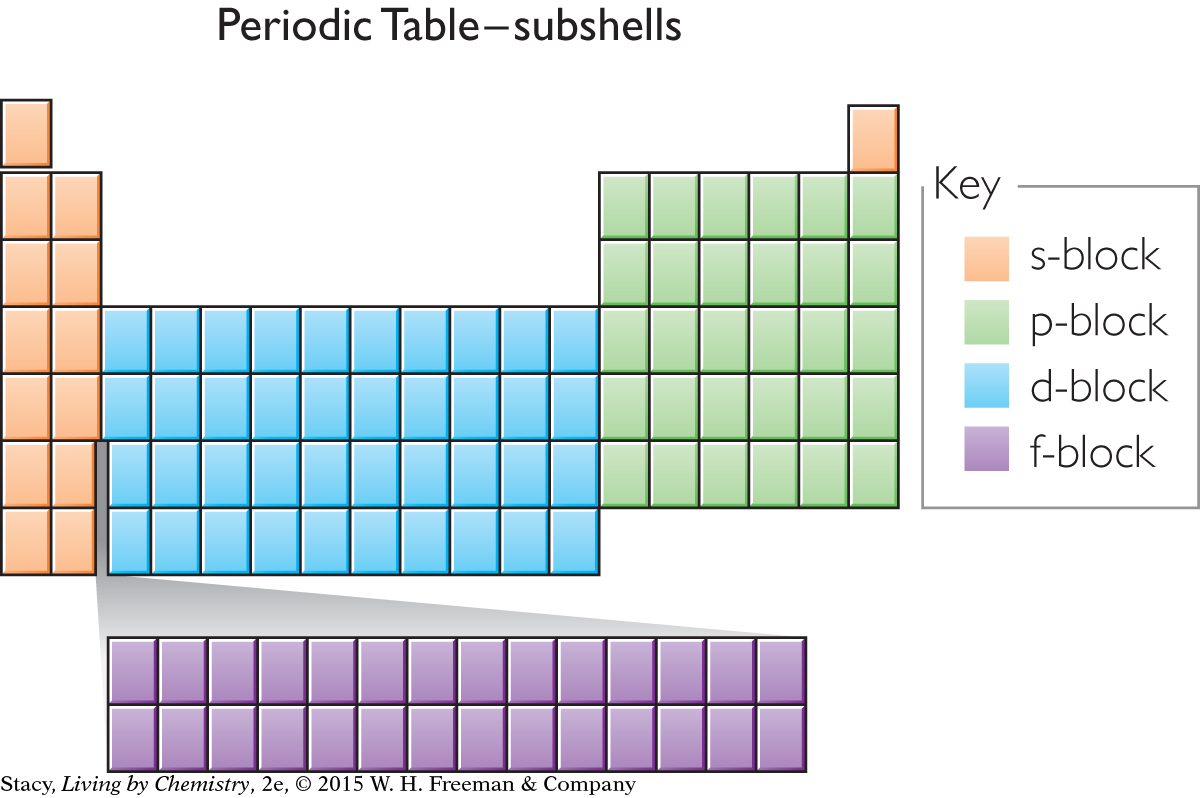
An outline of the periodic table appears here with color-coding to show the subshell for the outermost electron of each element. For example, any element located in the green area will have its outermost electron(s) in a p subshell.
As you proceed across the periodic table from one element to the next, one additional proton and one additional electron are added, along with one or more neutrons. Each additional electron goes into a specific subshell. If an atom is located in the orange areas of the table, the last electron is placed into an s subshell. If an atom is located in the blue area of the table, the last electron is placed into a d subshell. And so on.
The elements in each block have related properties. The elements in the s-block are reactive metals. The elements in the d-block tend to form colorful compounds that are used as pigments. The elements in the p-block tend to form colorless compounds.
DECODING THE TABLE
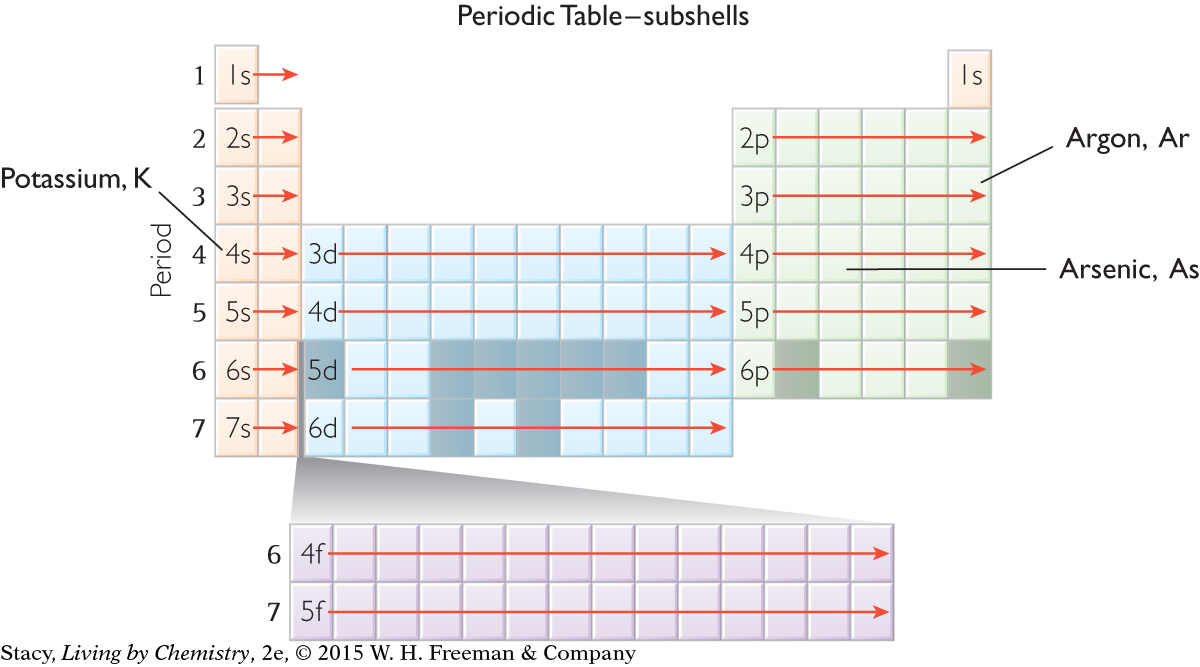
To write out an electron configuration for a specific element, you can simply “read” from the periodic table, moving across from left to right and then down to the next row. For example, the sequence of subshells for argon, Ar, is 1s, 2s, 2p, 3s, 3p. The electron configuration for argon is 1s22s22p63s23p6.
119
Important to Know
The s subshells fill with electrons before the d subshells from the previous shell. For example, the 4s subshell fills before the 3d subshell, the 5s subshell fills before the 4d subshell, and so on.
Everything runs smoothly until you reach the fourth row of the periodic table. After argon, you might expect the next electron to be in the 3d subshell. However, this does not happen. The next element is potassium, K. Like the other elements in Group 1A, potassium has one electron in the s subshell. So, you place an electron in 4s before 3d. The electron configuration for potassium is 1s22s22p63s23p64s1. The electron configuration for arsenic is 1s22s22p63s23p64s23d104p3.
You may have noticed that you only have to look at the ending of each electron configuration to figure out the identity of the element associated with it. The ending provides you with the exact spot on the periodic table where you can find the element.
Example 3
Electron Configuration of Cobalt
Write the electron configuration for cobalt, Co.
Solution
Locate cobalt on the periodic table. It is element number 27 and is located in the fourth period of the periodic table.
Simply trace your finger across the periodic table of subshells, writing the subshells as you go. When you get to cobalt, stop writing. Every subshell up to the 4s subshell is completely filled. In addition, cobalt has seven electrons in the 3d subshell. The answer is 1s22s22p63s23p64s23d7.
You can check that you have the correct electron configuration by adding the superscript numbers to make sure there are 27 electrons.
Noble Gas Shorthand
Noble Gas Shorthand
120
Depending on the element, the electron configuration can be lengthy to write. Plus, each element just repeats the electron configuration of the previous element but adds one more electron.
Rather than repeat the same thing every time, chemists have devised a quicker way to write out electron configurations. They use the noble gas at the end of each period as a placeholder to symbolize all of the filled subshells before that place on the table. Using this “shorthand” method, the electron configuration of cobalt is [Ar]4s23d7.
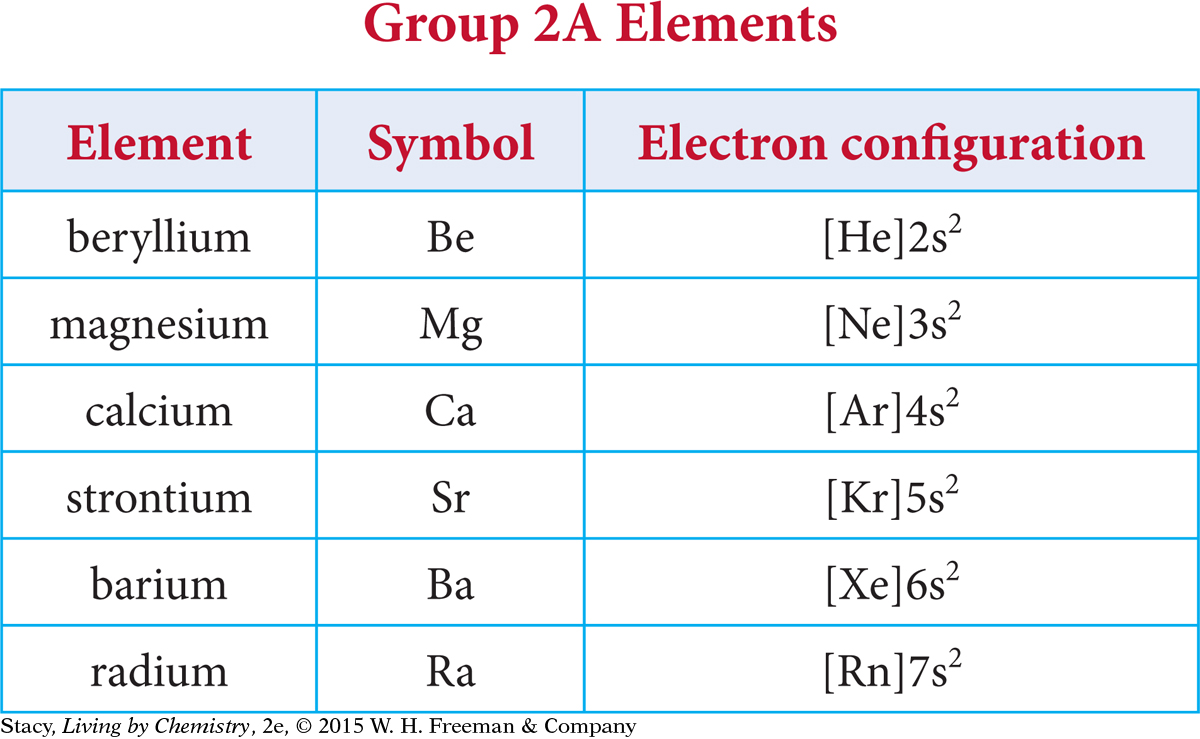
Shorthand notation allows you to make some interesting comparisons. Notice that the noble gas shorthand notation emphasizes the valence electrons. Using this method, it is easy to see that each element in Group 2A has two valence electrons, both located in an s subshell.
Example 4
Electron Configuration of Selenium
Find the element Selenium, Se, element number 34, on the periodic table.
What is the electron configuration of selenium?
Write the electron configuration using noble gas shorthand.
In what subshells are selenium’s valence electrons?
Solution
Selenium is in the p-block, in Period 4. The electron configuration of selenium is 1s22s22p63s23p64s23d104p4.
The noble gas that comes before selenium is argon, Ar. So, the noble gas shorthand for this configuration is [Ar]4s23d104p4.
Selenium’s valence electrons are in subshells 4s and 4p.
LESSON SUMMARY
LESSON SUMMARY
What does the periodic table indicate about the arrangements of electrons?
Electrons in atoms are arranged into basic shells labeled n = 1, 2, 3, and so on. These shells are divided into subshells. The number of subshells in each shell is equal to n. The subshells are referred to as s, p, d, and f subshells. The s, p, d, and f subshells can have a maximum of 2, 6, 10, and 14 electrons, respectively. Chemists use electron configurations to specify the arrangements of electrons in subshells. The periodic table provides the information needed to write electron configurations.
KEY TERM
electron configuration
121
Exercises
Reading Questions
What are electron subshells?
What is an electron configuration?
How is the arrangement of electrons in an atom related to the location of the atom on the periodic table?
Reason and Apply
How many subshells are in each shell: n = 1, n = 2, n = 3, n = 4?
What is the total number of subshells for elements in Period 5 of the periodic table?
Draw a subshell model for each of these elements, putting the electrons in their appropriate places.
sodium, Na
neon, Ne
carbon, C
vanadium, V
What is the outermost subshell for bromine, Br?
Name an element with electrons in the f subshell.
Consider the element with the atomic number 13.
What is the electron configuration for the element with atomic number 13?
How many valence electrons does element number 13 have? How do you know?
How many core electrons does element number 13 have? How did you figure that out?
Explain why the chemical properties of argon, krypton, and xenon are similar, even though there are 18 elements between argon and krypton and 32 elements between krypton and xenon.
Write the electron configuration for each of these atoms. Then write it using the noble gas shorthand method.
oxygen
chlorine
iron
calcium
magnesium
silver
silicon
mercury
You should be able to figure out the identity of an atom from its electron configuration alone. Describe at least two ways you could do this.
Which elements are described by these electron configurations?
1s22s22p63s23p64s23d4
1s22s22p63s23p2
1s22s22p3
1s22s22p63s23p64s23d104p65s24d105p66s1
1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p2
[Kr]5s24d9