Featured LAB: Electroplating Metals
Featured LAB
134
Electroplating Metals
!
SAFETY
Instructions
Wear safety goggles at all times.
The solution contains acid. Handle carefully. Rinse the nickel strips after they have been in the copper solution.
Purpose
To use electrochemistry to extract metals from ionic compounds in a solution.
Procedure
Set up the electroplating apparatus as shown. Observe what happens.
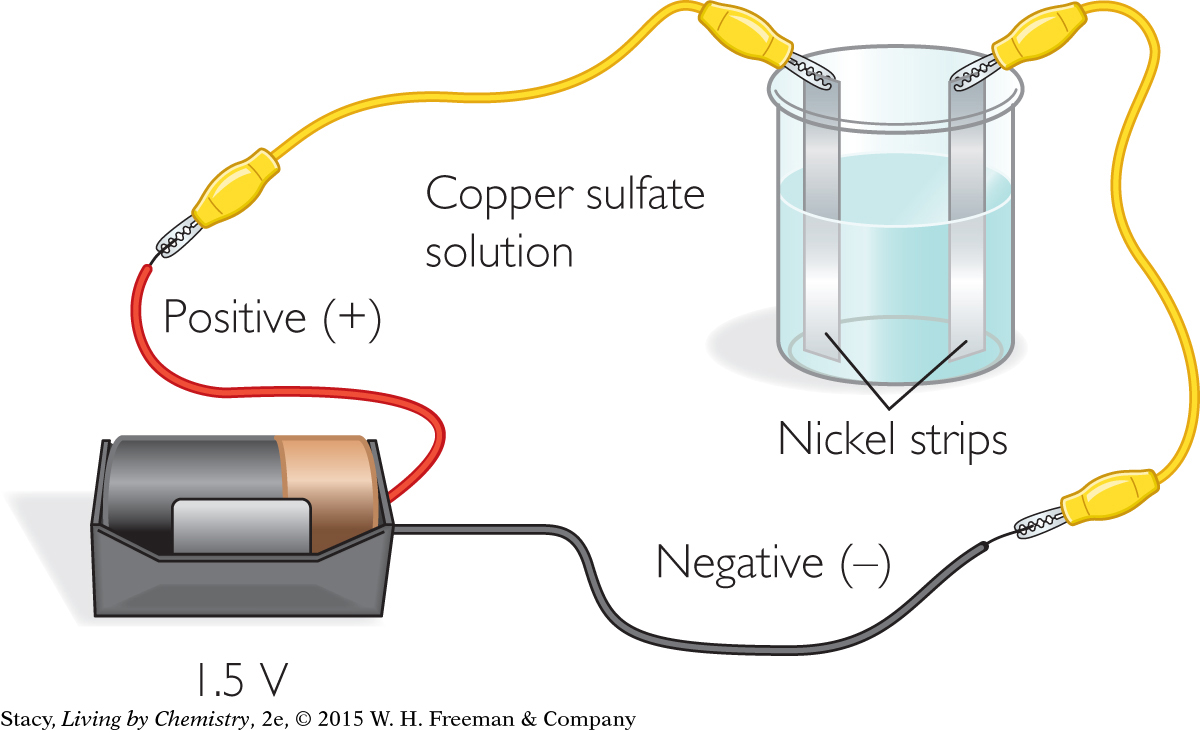
Materials
copper sulfate plating solution
250 mL beaker
2 nickel strips (cut into about 1-by-3-in. strips)
1.5-volt D-cell battery with holder
2 insulated wires with alligator clips
Switch the sides of the battery to which the two alligator clips are attached. Wait at least one minute or until you notice a change.
Reverse the wiring back to its original position.
Observations
What did you observe when you hooked up the nickel strips to the battery?
What happened when you reversed the flow of electricity?
Where does the copper come from that ends up on the nickel strip?
What is in the copper sulfate solution?
Write a short paragraph explaining your observations.
Making Sense Are copper atoms and copper ions the same element? Explain your thinking.
If You Finish Early Consider a sample of gold chloride, AuCl3. Explain what procedure you might follow in order to extract solid gold from the compound.