Chapter 5 Summary

CHAPTER 5
Building with Matter
SUMMARY
139
KEY TERMS
dissolve
soluble
insoluble
conductivity
chemical bond
ionic bonding
molecular covalent bonding
metallic bonding
network covalent bonding
covalent bonding
molecule
electroplating
Alchemy Update
How can substances with new properties be made?
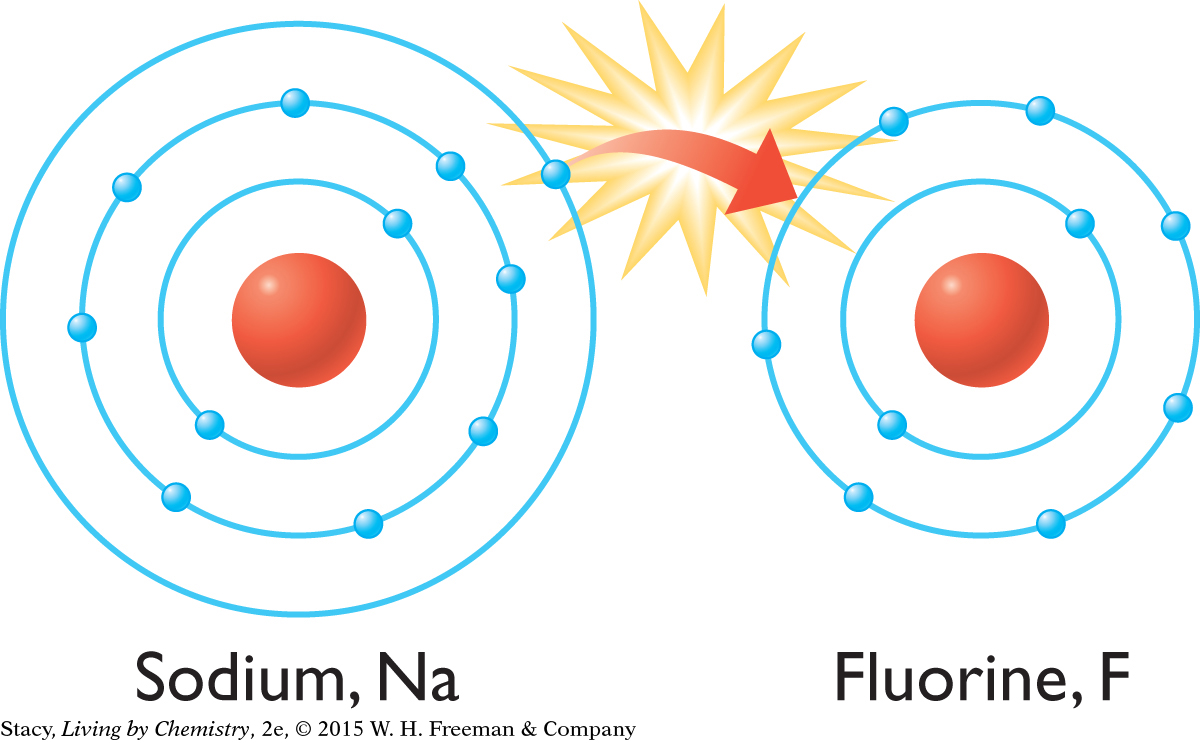
A bond is an attraction between the nucleus of one atom and the electrons of another atom. When atoms bond together, new substances with new properties are made. Conversely, elements can be extracted from compounds by breaking the bond.
There are four models of bonding, depending on the location of electrons within the atoms in the substance: ionic, molecular covalent, metallic, and network covalent. Certain properties are associated with each type of bonding.
Atoms form bonds with other atoms, creating a variety of substances with new properties. It is possible to make compounds that look like or behave like gold, such as iron pyrite, FeS2, also called fool’s gold. Also, you can use chemical bonding to make substances that are even more valuable than gold.
REVIEW EXERCISES
Question 5.1
1. What does the phrase “as good as gold” mean as it applies to this class?
Question 5.2
2. Describe how you could experimentally determine the type of bonding present in a substance.
Question 5.3
3. Predict whether isopropanol, C3H8O(l), will conduct electricity. State your reasoning.
Question 5.4
4. Make a table like this one. Fill it with the model of bonding that fits the category.
