Chapter 6 Summary
183

CHAPTER 6
Speaking of Molecules
SUMMARY
KEY TERMS
molecular formula
structural formula
isomer
HONC 1234 rule
Lewis dot structure
Lewis dot symbol
bonded pair
lone pair
octet rule
double bond
triple bond
functional group
chemical reaction
synthesis
chemical equation
reactant
product
catalyst
Smells Update
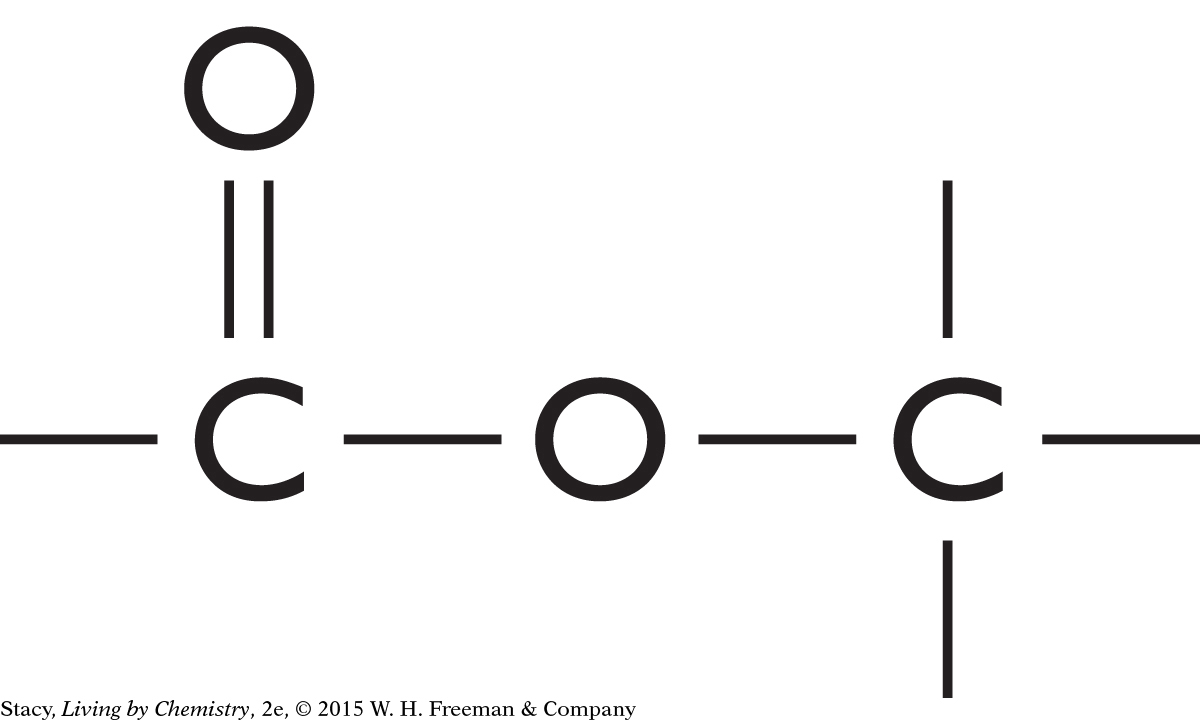
The investigation into the chemistry of smell has challenged you to explore how molecules are put together. So far you know that the smell of a substance is related to its molecular formula, chemical name, and any functional groups present.
This is based on the data investigated so far. Further investigation may lead you in new directions.
REVIEW EXERCISES
Question 6.1
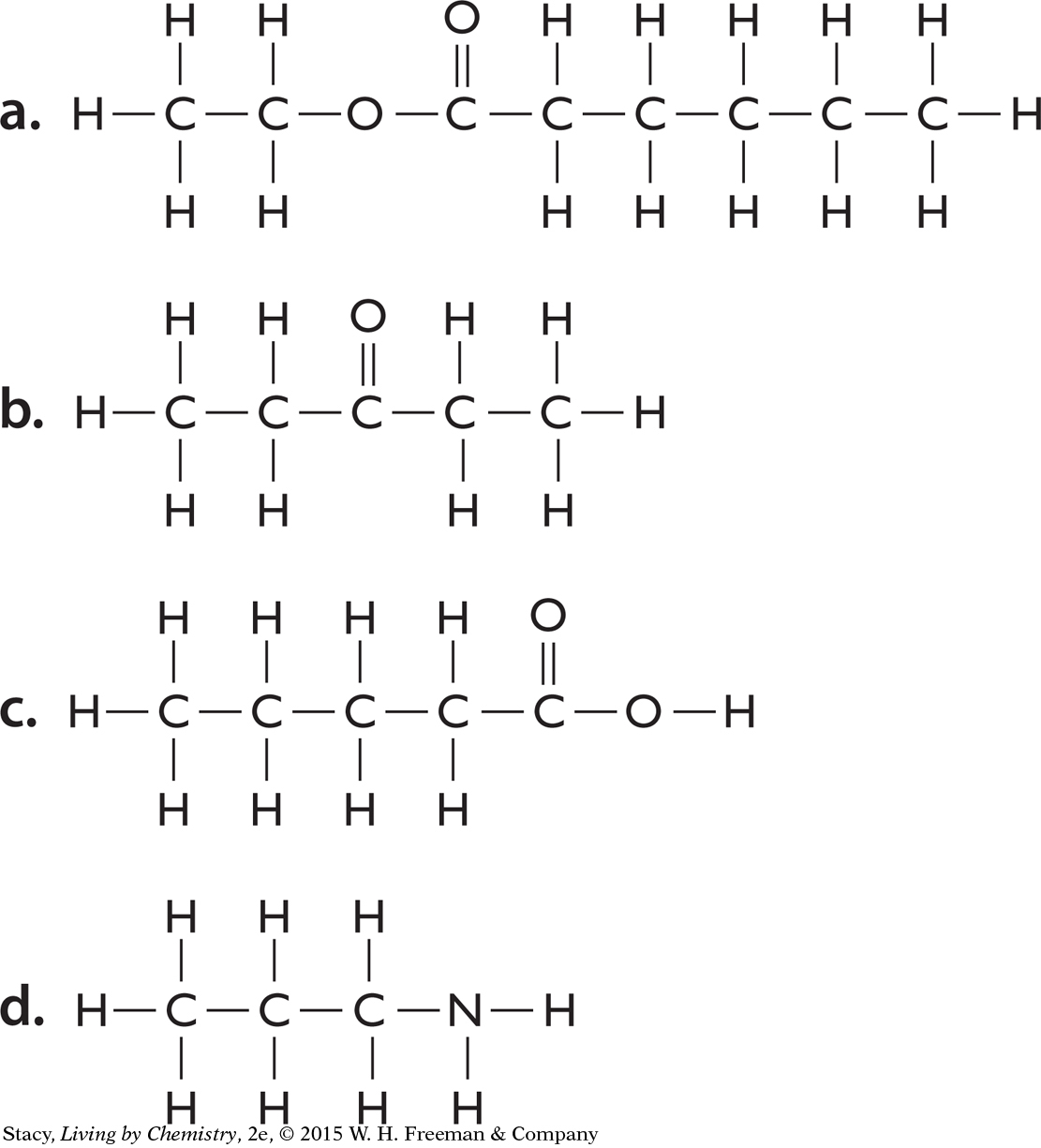
1. What functional groups are present in each of these molecules?
Question 6.2
2. Draw the Lewis dot structure and the structural formula for each of these molecules.
SiF4
CO2
CH4
SF2
C2H4
C2H2
C2H6
Question 6.3
3. How many lone pairs are in each of these molecules?
CO2
SiF4
CH4
Question 6.4
4. Draw at least two structural formulas for the molecular formula C3H6O.
Question 6.5
5. From what you’ve learned so far, how is molecular structure related to smell?