Featured ACTIVITY: Connect the Dots
Featured ACTIVITY
160
Connect the Dots
Purpose
To investigate the role of electrons in covalent bonding.
Materials
Lewis dot puzzle pieces
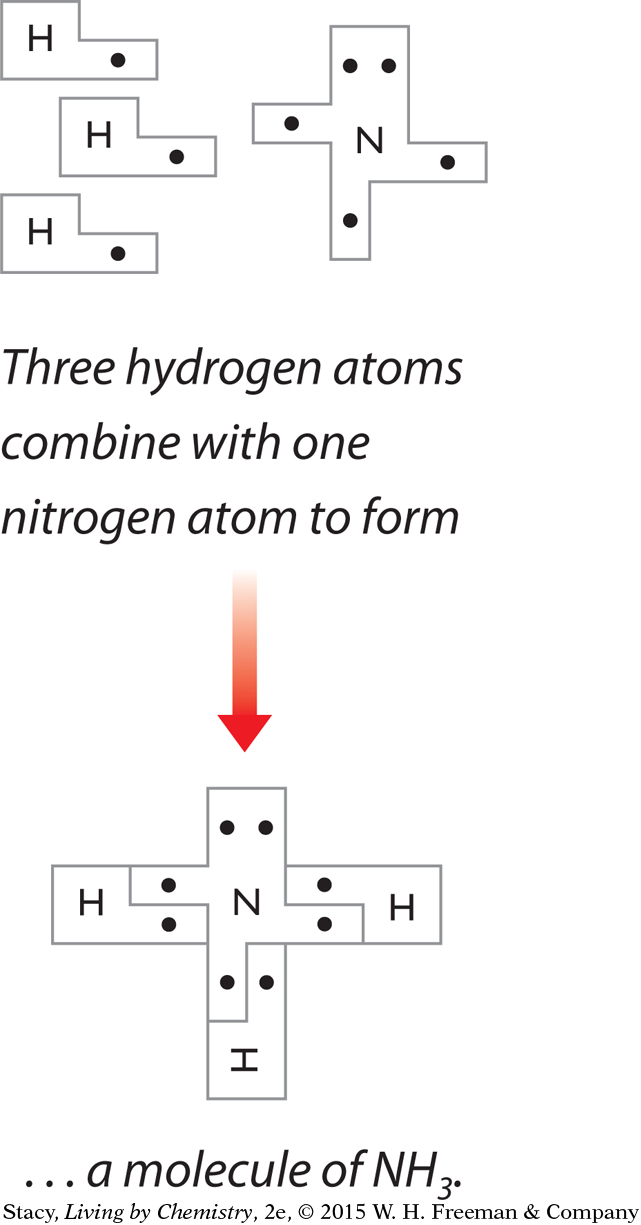
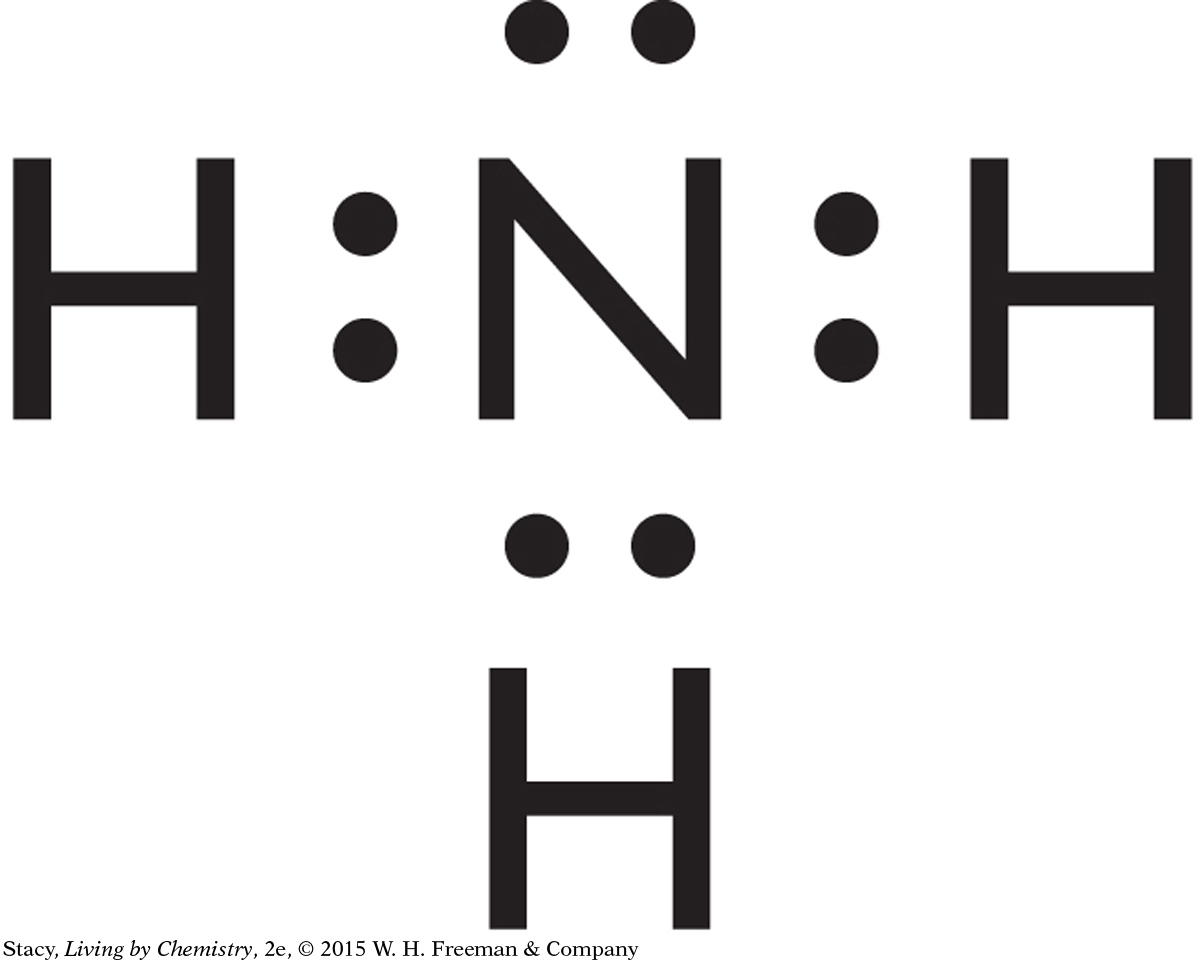
Instructions
The puzzle pieces you have been given represent Lewis dot symbols. The puzzle pieces allow you to pair up electrons and create molecules.
Use the puzzle pieces to construct the molecules given below. Then draw the Lewis dot structure for each molecule.
PH3 HOCl F2 CH3Cl Use the puzzle pieces to create more molecules, following the directions given below. For each molecule, draw the Lewis dot structure and write the molecular formula.
Use one S atom and as many H atoms as you need.
Use one Si atom and as many F atoms as you need.
Use two O atoms and as many H atoms as you need.
Use the puzzle pieces to construct a molecule with the molecular formula C2H6. Draw its Lewis dot structure and its structural formula.
Use the puzzle pieces to construct all the possible isomers of C3H8O. Draw Lewis dot structures for each isomer. Do the molecules follow the HONC 1234 rule?
Use the puzzle pieces to design your own molecule with at least five carbon atoms. Draw its Lewis dot structure. What is the molecular formula of your designer molecule? Does it obey the HONC 1234 rule?
Find a puzzle piece for each type of atom. Put hydrogen and helium aside. Sort the rest of the puzzle pieces according to the periodic table. Record your sort by copying it into a grid. Include the symbols and the dots.
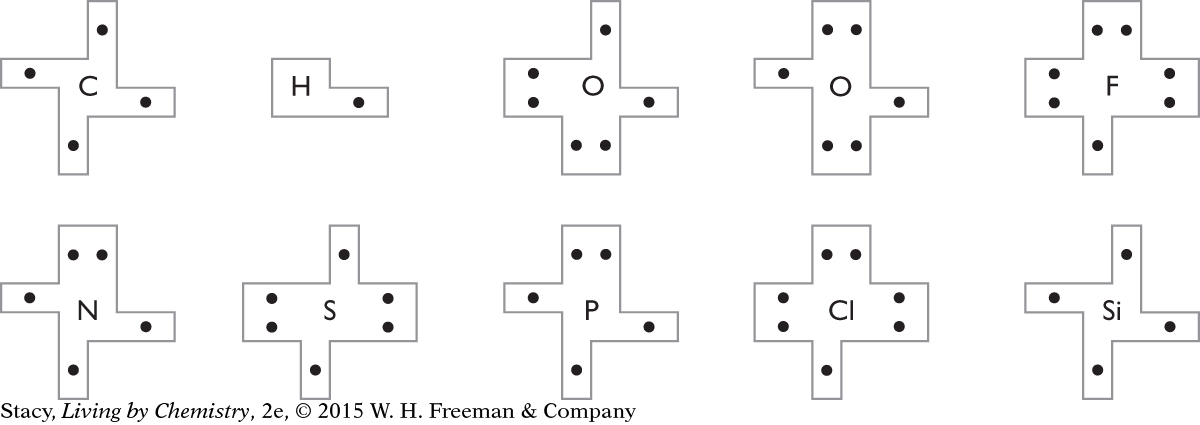
List two patterns that you notice.
Making Sense Using what you’ve learned, explain why the HONC 1234 rule works.
If You Finish Early Draw the Lewis dot structures of two different molecules with the molecular formula C2H7N.