LESSON 31: Connect The Dots: Lewis Dot Symbols
161
THINK ABOUT IT
The HONC 1234 rule is a great trick to help you figure out the structures of molecules. But why does it work? Why does carbon connect with four atoms, while hydrogen can connect with only one atom? To answer these questions, let’s take a closer look at bonds.
How does one atom bond to another in a molecule?
To answer this question, you will explore
The Covalent Bond
Lewis Dot Symbols and Structures
Bonded Pairs and Lone Pairs
The Covalent Bond
EXPLORING THE TOPIC
The Covalent Bond
All of the smelly compounds we have studied so far have been molecules. In fact, most of the substances that smell are made up of molecules, not ionic, metallic, or network covalent compounds.
The bonds that are found in molecules are called covalent bonds. Covalent bonds are formed between the atoms of nonmetallic elements. There are only about 15 nonmetallic elements in the periodic table. That is not many compared to the number of metallic elements.
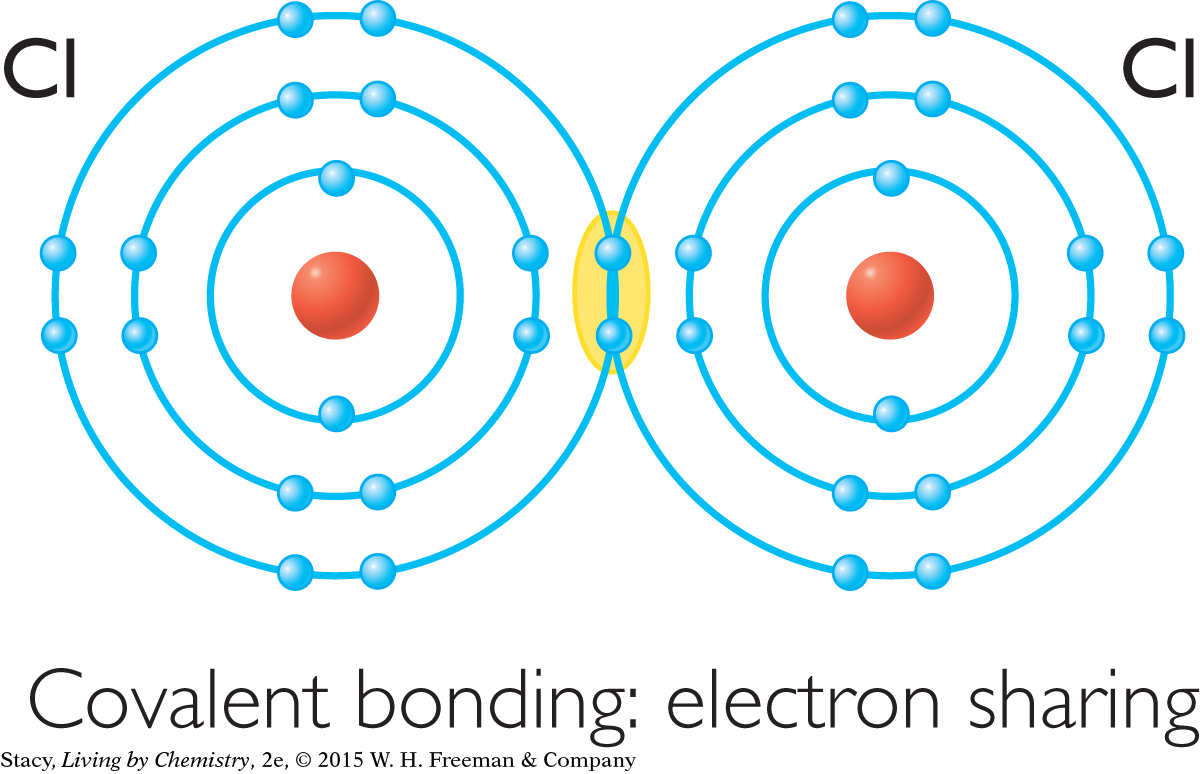
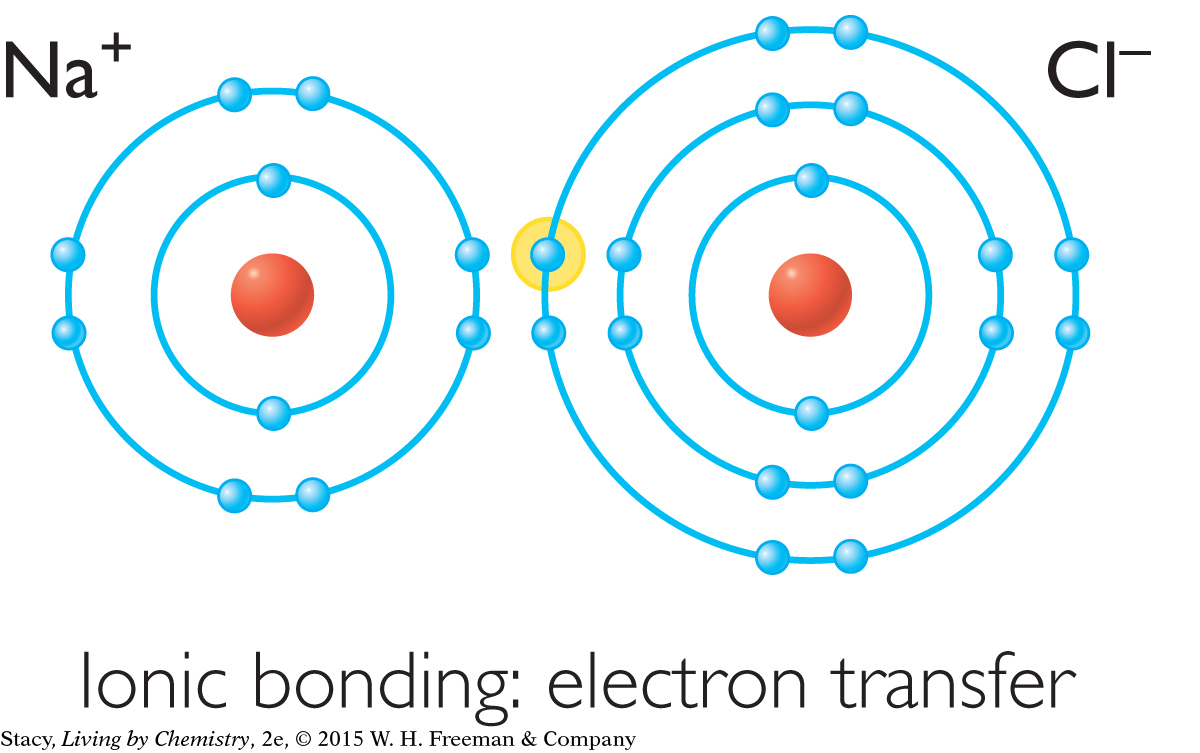
The atoms involved in a covalent bond share a pair of valence electrons between them. The drawing below shows the difference between covalent bonds and ionic bonds.
|
|
In covalent bonds, the nonmetal atoms are sharing electrons. As a result, the nonmetal atoms are tightly bound together, but the atoms do not become ions with charges.
Big Idea
Big Idea
A covalent bond is one in which nonmetal atoms share one or more pairs of electrons with one another. An ionic bond is one in which a metal atom gives up electrons to a nonmetal atom.
Lewis Dot Symbols and Structures
Lewis Dot Symbols and Structures
162
Structural formulas are one way to show the structure of a molecule on paper. Each line in a structural formula can be replaced with a pair of dots to represent the electrons that are being shared.
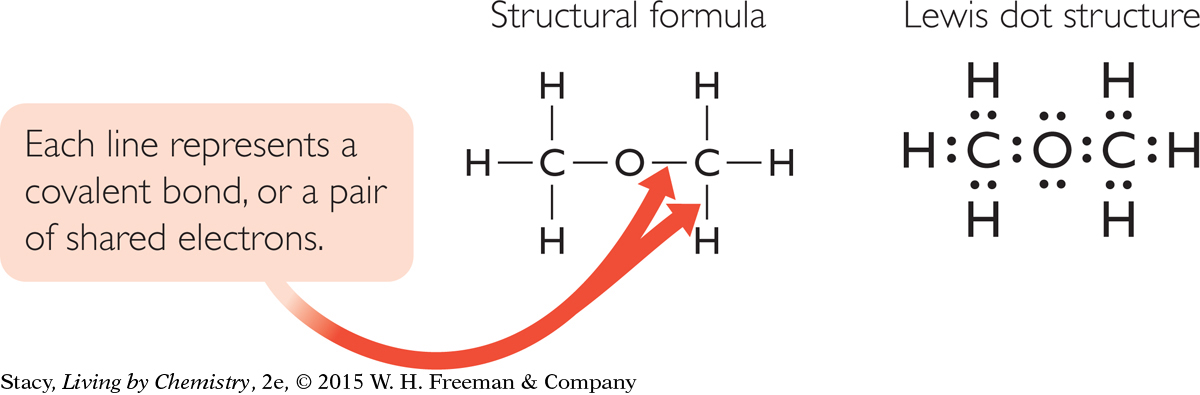
A drawing of a molecule that uses dots to represent the valence electrons is called a Lewis dot structure. Above is a molecule of dimethyl ether, C2H6O. Both the structural formula and the Lewis dot structure represent the same molecule.
Lewis dot structures keep track of every valence electron in every atom of a molecule. If you break the molecule apart into its individual atoms, you can see where each valence electron came from.
CONSUMER CONNECTION
CONSUMER
CONNECTION
The smell of ammonia is powerful and irritating. Ammonia is toxic and can damage the interior of your nose and lungs. However, solutions with dissolved ammonia make good household cleansers. And small amounts of ammonia are used in smelling salts to revive a person who has fainted.

A Lewis dot symbol is the symbol of an element with dots to show the number of valence electrons that a single atom of that element has. Examine these Lewis dot symbols for hydrogen, oxygen, nitrogen, and carbon. Notice that some electrons are not paired up. These electrons are referred to as unpaired electrons and are available for bonding. The unpaired electrons form covalent bonds with other atoms. Lewis dot symbols can help to explain the HONC 1234 rule and the bonding of nonmetal atoms.
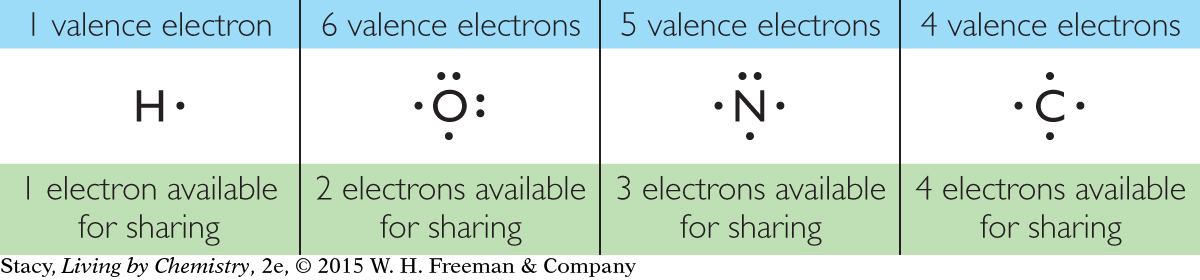
Recall that you can determine the number of valence electrons in an atom by locating its element on the periodic table. Hydrogen is in Group 1A and has one valence electron. Carbon is in Group 4A and has four valence electrons. Once you know the number of valence electrons an atom has, it is easy to draw a Lewis dot symbol for it.
The Lewis dot symbol is named after Gilbert Newton Lewis, a chemist who was a professor at the University of California at Berkeley. Lewis first introduced the idea of shared electrons and covalent bonds in 1916. He was the first scientist to use a system of dots to represent valence electrons in atoms.
Bonded Pairs and Lone Pairs
Bonded Pairs and Lone Pairs
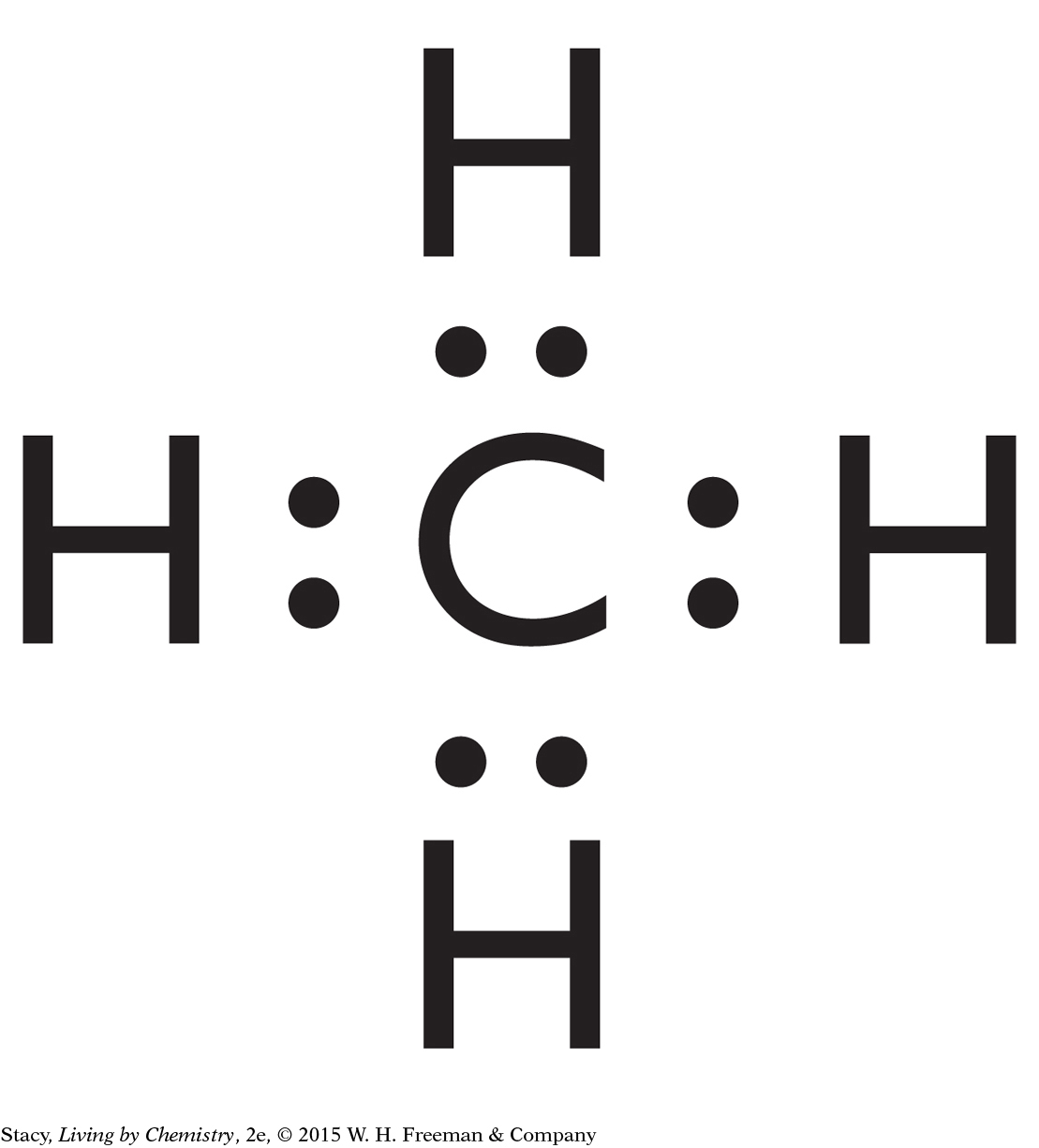
Lewis dot symbols can be used to draw Lewis dot structures and structural formulas for molecules. Imagine bringing together the Lewis dot symbols for hydrogen and carbon to form methane, CH4.
163
Because carbon bonds four times, it will bond with four hydrogen atoms. Once they are bonded, every valence electron in all of the atoms is paired up. The pairs of electrons between atoms are called bonded pairs. There are four bonded pairs in the methane molecule shown.
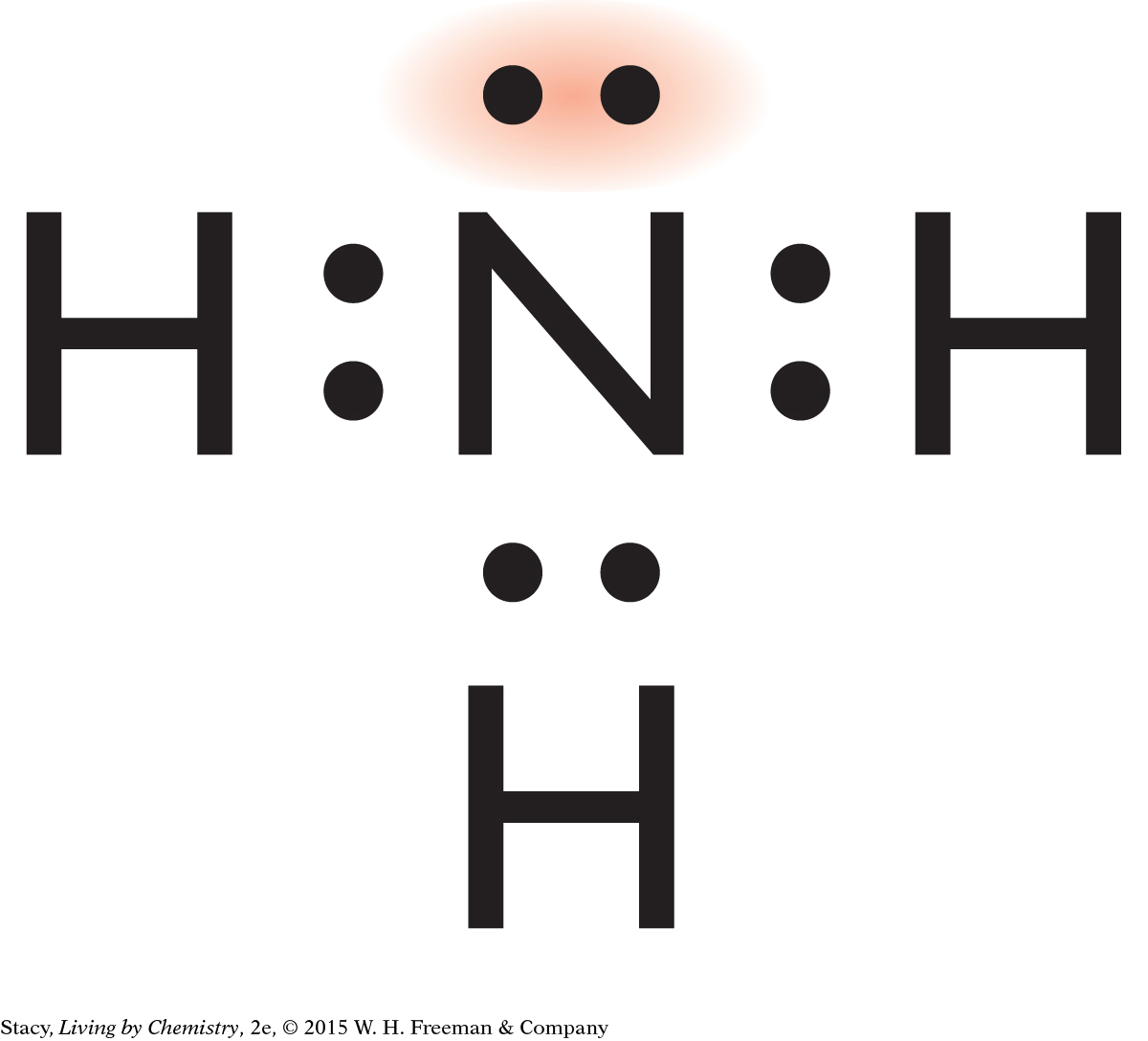
A pair of electrons in a molecule that are not shared between atoms is called a lone pair of electrons. In a molecule of ammonia, the nitrogen atom has one lone pair of electrons.
Example
Lewis Dot Structure of PCl3
Examine the Lewis dot structure of PCl3, phosphorus trichloride.

Draw the Lewis dot symbols for phosphorus and chlorine.
How many bonds does a phosphorus atom make? How many does each chlorine atom make?
How many bonded pairs does this molecule have? How many lone pairs?
How many covalent bonds does this molecule have?
Solution
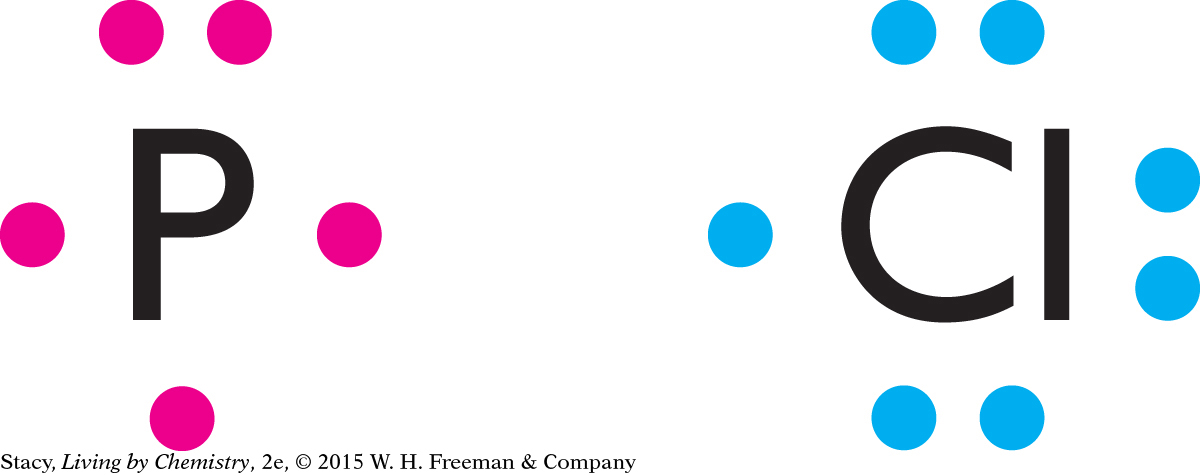
The periodic table can help you figure out how many valence electrons phosphorus and chlorine have. Phosphorus is in Group 5A and chlorine is in Group 7A, so they have five and seven valence electrons, respectively.
Phosphorus has three unpaired electrons, so it makes three bonds. Chlorine has only one unpaired electron, so it makes one bond.
 The three bonded pairs of electrons are circled. All of the other pairs of electrons are lone pairs.
The three bonded pairs of electrons are circled. All of the other pairs of electrons are lone pairs.The molecule has three bonded pairs and ten lone pairs.
Each bonded pair represents one covalent bond. There are three covalent bonds in the molecule.
LESSON SUMMARY
LESSON SUMMARY
How does one atom bond to another in a molecule?
KEY TERMS
Lewis dot structure
Lewis dot symbol
bonded pair
lone pair
Molecules are made of nonmetal atoms that are covalently bonded. A covalent bond is a bond in which a pair of electrons is shared by two atoms. Lewis dot symbols keep track of the number of valence electrons in each atom. They can help you to predict covalent bonding between atoms. A Lewis dot structure shows how the atoms in an entire molecule are bonded together. The valence electrons that are involved in bonding are called a bonded pair. The valence electrons that are paired up in a molecule, but do not take part in bonding, are called lone pairs.
164
Exercises
Reading Questions
How are an ionic bond and a covalent bond different? Similar?
What is a Lewis dot structure? Explain how you would create one.
Reason and Apply
Draw the Lewis dot symbols for these elements:
Te I K Bi In Pb Arrange them in order of their group numbers on the periodic table.
Determine how many covalent bonds each element would make.
Germanium, antimony, selenium, and bromine each bond to a different number of hydrogen atoms.
GeH4 SbH3 H2Se HBr Draw Lewis dot symbols for Ge, Sb, Se, and Br.
Draw a Lewis dot structure for each molecule.
Explain the pattern in the number of hydrogen atoms.
Draw Lewis dot structures for the molecules listed here.
TeCl2
HI
AsBr3
SiF4
F2
How many lone pairs does each of the molecules in Exercise 5 have?
In your own words, explain why the HONC 1234 rule works.
Draw Lewis dot puzzle pieces for Si, P, S, and Cl. What rule would you make for Si, P, S, and Cl? What would be the name of this bonding rule?