Chapter 7 Summary

CHAPTER 7
Building Molecules
SUMMARY
KEY TERMS
ball-and-stick model
tetrahedral shape
electron domain
electron domain theory
pyramidal shape
bent shape
trigonal planar shape
linear shape
space-filling model
receptor site theory
212
Smells Update
The smell of a substance sometimes has more to do with molecular shape than with the functional groups that are present. In this chapter, you learned about three-dimensional molecular structure and related it to smell. Ball-and-stick and space-filling models show how atoms are arranged within a molecule. The actual shape of a molecule is determined by the locations of electron domains.
No matter what shape a molecule has, it must fit into an appropriate receptor site in order for the nose to detect its smell.
REVIEW EXERCISES
Question 7.1
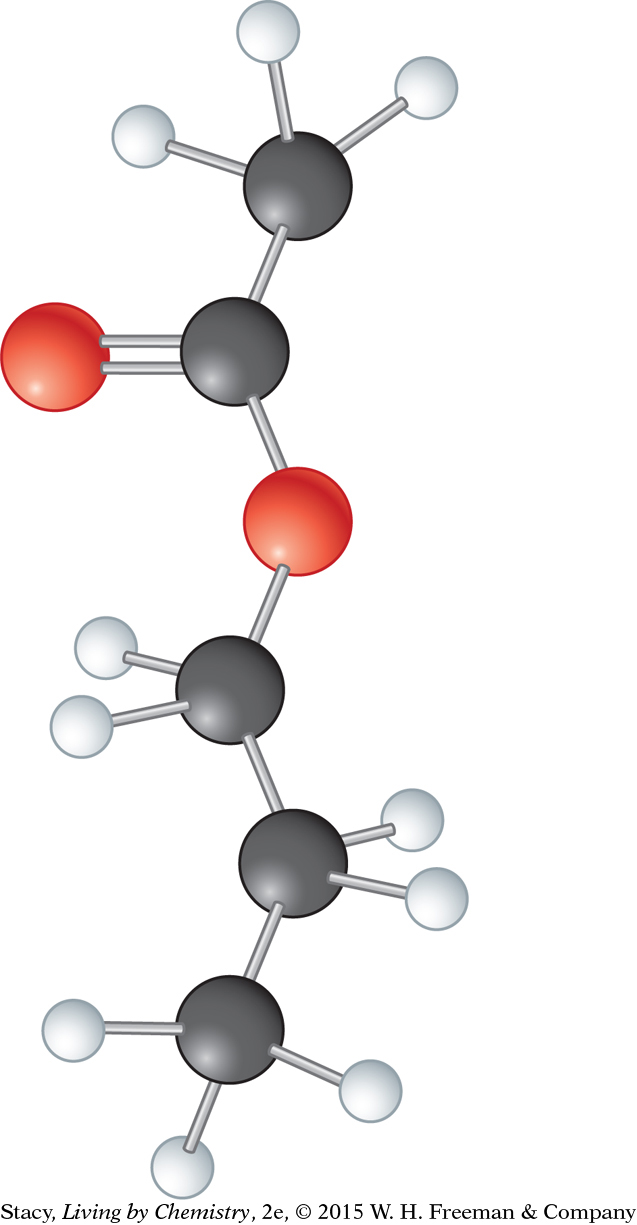
1. Examine the ball-and-stick model for propyl acetate.
What is the molecular formula for this molecule?
Draw the Lewis dot structure and the structural formula.
What functional group is in the molecule?
Predict the smell of the compound.
Question 7.2
2. Predict the shape of each molecule. Support your answer.
ammonia, NH3
silicon chloride, SiCl4
hydrogen sulfide, H2S
hydrogen cyanide, HCN
formaldehyde, CH2O
Question 7.3
3. Explain what determines a molecule’s shape.
Question 7.4
4. Draw a possible Lewis dot structure for C4H10O.
Question 7.5
5. From what you’ve learned so far, how is molecular shape related to smell?