LESSON 36: New Smells, New Ideas: Ball-and-Stick Models
185
THINK ABOUT IT
The structural formula is a valuable source of information about a molecule. You can use the structural formula to identify functional groups in a molecule. Knowing the functional group also helps you to predict molecular properties, including smell. However, sometimes only knowing the functional group is not enough to predict the smell of a compound.
What three-dimensional features of a molecule are important in predicting smell?
To answer this question, you will explore
New Smell Molecules
Three-Dimensional Models
New Smell Molecules
EXPLORING THE TOPIC
New Smell Molecules
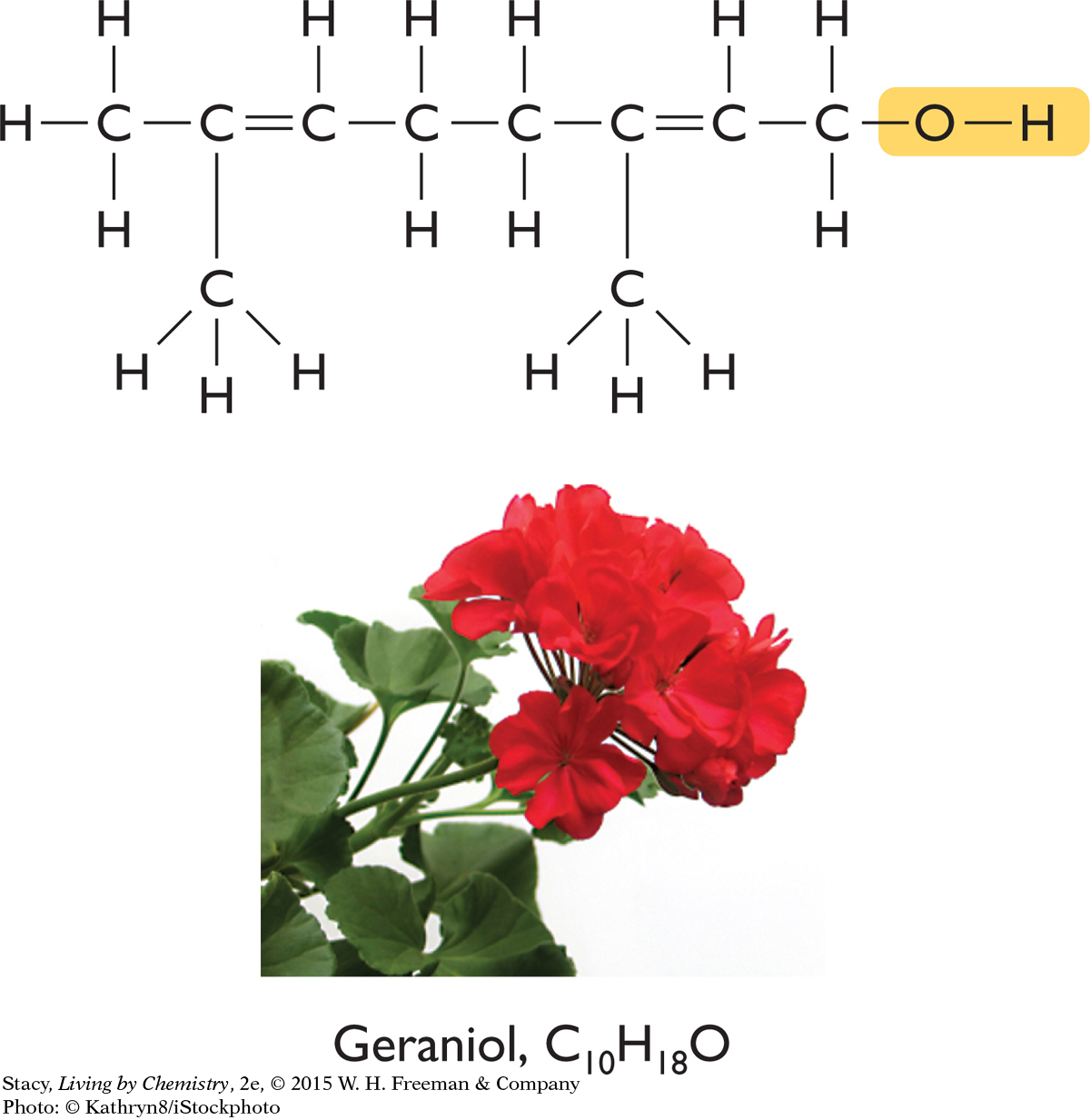
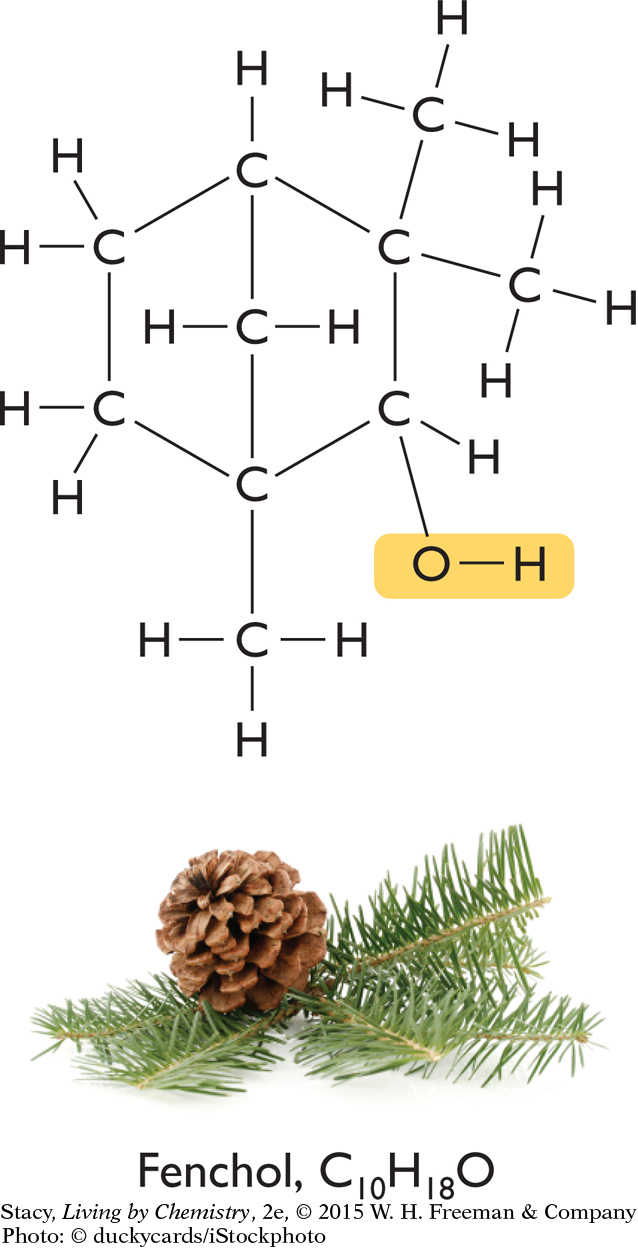
Below are three compounds, each with a distinctive smell. In spite of their wide range of smells, each of the three compounds contains the same functional group.
© Kathryn8/iStockphoto
|

© Helen Sessions/Alamy
|
© duckycards/iStockphoto
|
Take a moment to locate their functional groups. Each of these molecules has a hydroxyl functional group, and each name ends in “-ol.” All three molecules are alcohols. So, why are the three smells so different? Something besides just functional group affects the smell of a compound. The answer lies in the three-dimensional shape of the molecules of each compound.
Three-Dimensional Models
Three-Dimensional Models
186
It is difficult to show a three-dimensional drawing of a molecule on a flat piece of paper. With a molecular model set, you can build a three-dimensional structure.
CONSUMER CONNECTION
CONSUMER
CONNECTION
The fragrance industry uses more than 5000 compounds. When creating a scented product, chemists must consider how the compounds interact with each other and with the human body. Fragrance chemicals enter the human body through the skin and by ingestion, so scents must be made safe in addition to pleasant-smelling.

Notice that the bonds in a model kit look like little sticks. And the atoms are small spheres. These models are called ball-and-stick models.
A picture of a ball-and-stick model for ethyl acetate, C4H8O2, is shown here. The carbon atoms are shown in black, the hydrogen atoms are white, and the oxygen atoms are red.
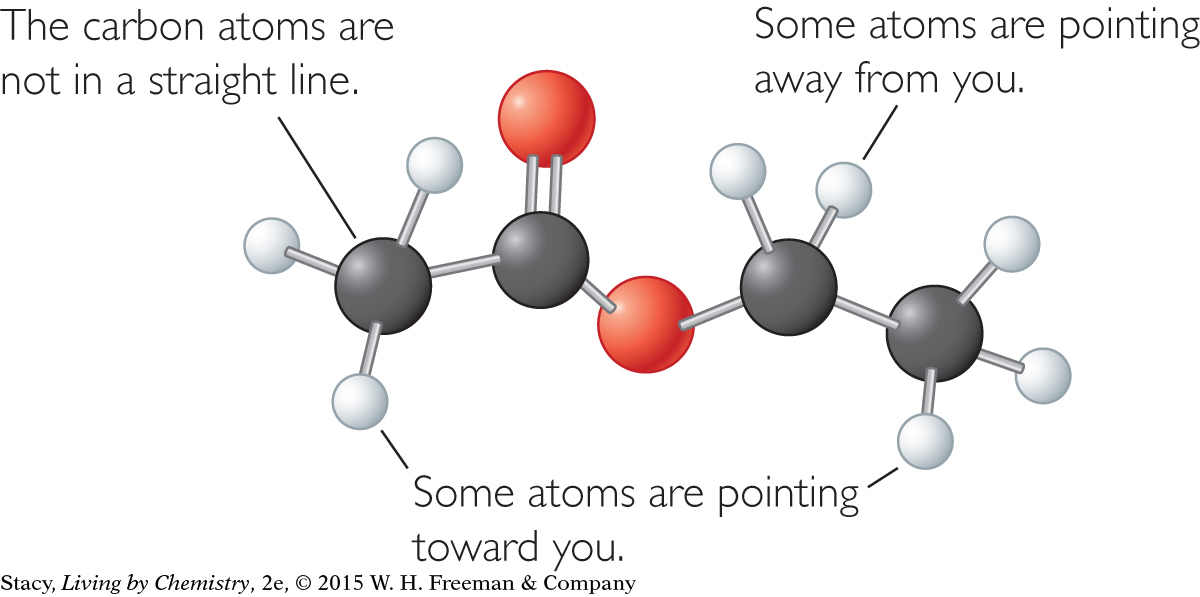
Illustrations of ball-and-stick models have some drawbacks, in that some atoms may be partially visible or entirely hidden. However, the illustrations convey more information about molecular shape than structural formulas do. Using a real model is the best way to examine the three-dimensional shape of a molecule. The two representations for a molecule of citral, C10H16O, are shown for comparison. Look for similarities and differences between them.
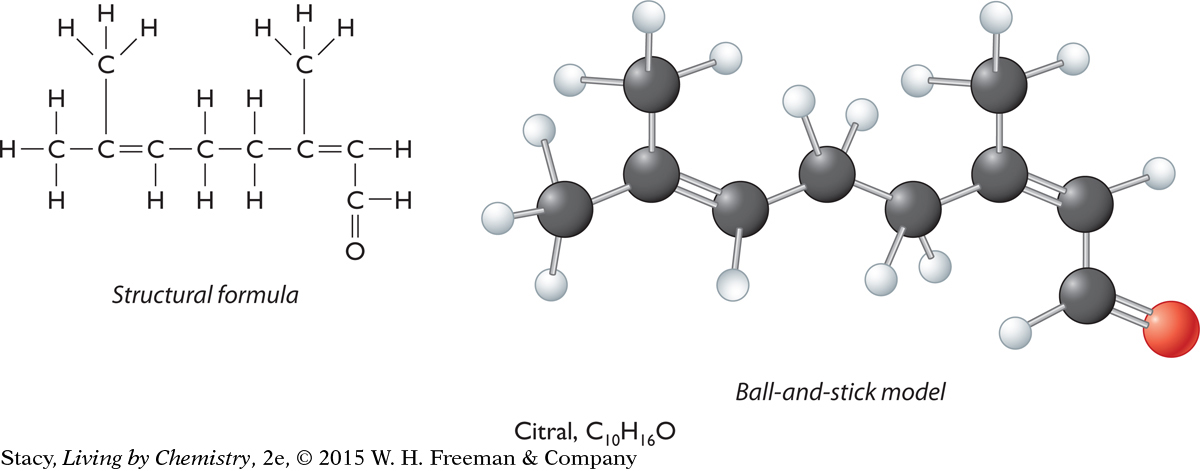
187
HISTORY CONNECTION
HISTORY
CONNECTION
Abu Ali ibn Sina, also known as Avicenna, was born in 980 C.E. in what is now Uzbekistan—then Persia. He became a physician when he was 18. Besides making pioneering contributions to modern medicine, Ibn Sina developed the process of extracting essential oils from plants through distillation. He worked with rose water, which became a popular fragrance and led others to experiment with other flowers.

Notice that the structural formula shows the citral molecule as flat with all of the carbon atoms in a line. The ball-and-stick representation, on the other hand, shows that the carbon atoms are not arranged in a line, but are connected in a zigzag fashion. Both representations contain much of the same information, but the ball-and-stick model adds information about the way the atoms are arranged in space.
Example
Ball-and-Stick Model
Examine the drawing of this ball-and-stick model of isopentylacetate, C7H14O2.
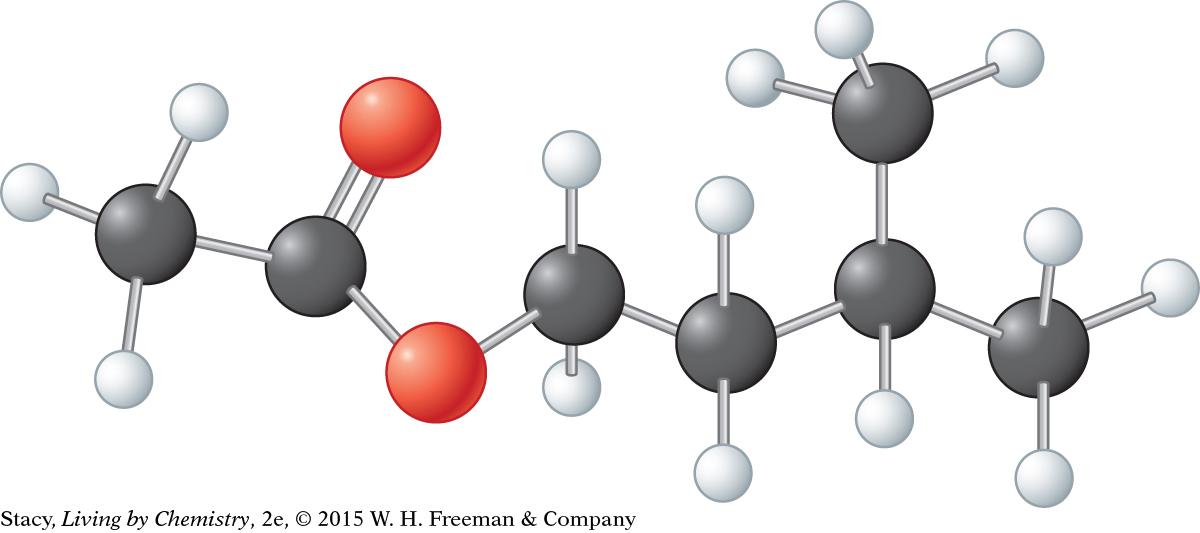
Draw the structural formula of this compound.
What functional group is in the compound?
Predict a smell for this compound.
Solution
The structural formula of this compound is
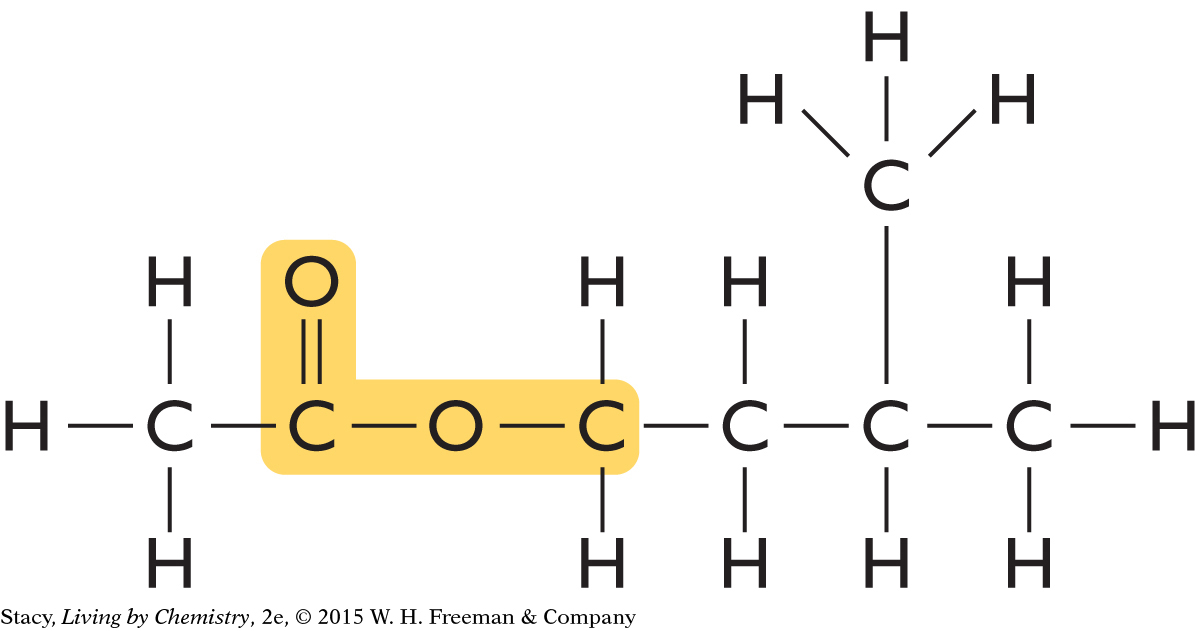
This compound has an ester functional group.
Because it has an ester group and its name ends in “-ate,” it probably smells sweet. (In fact, this molecule smells like bananas.)
188
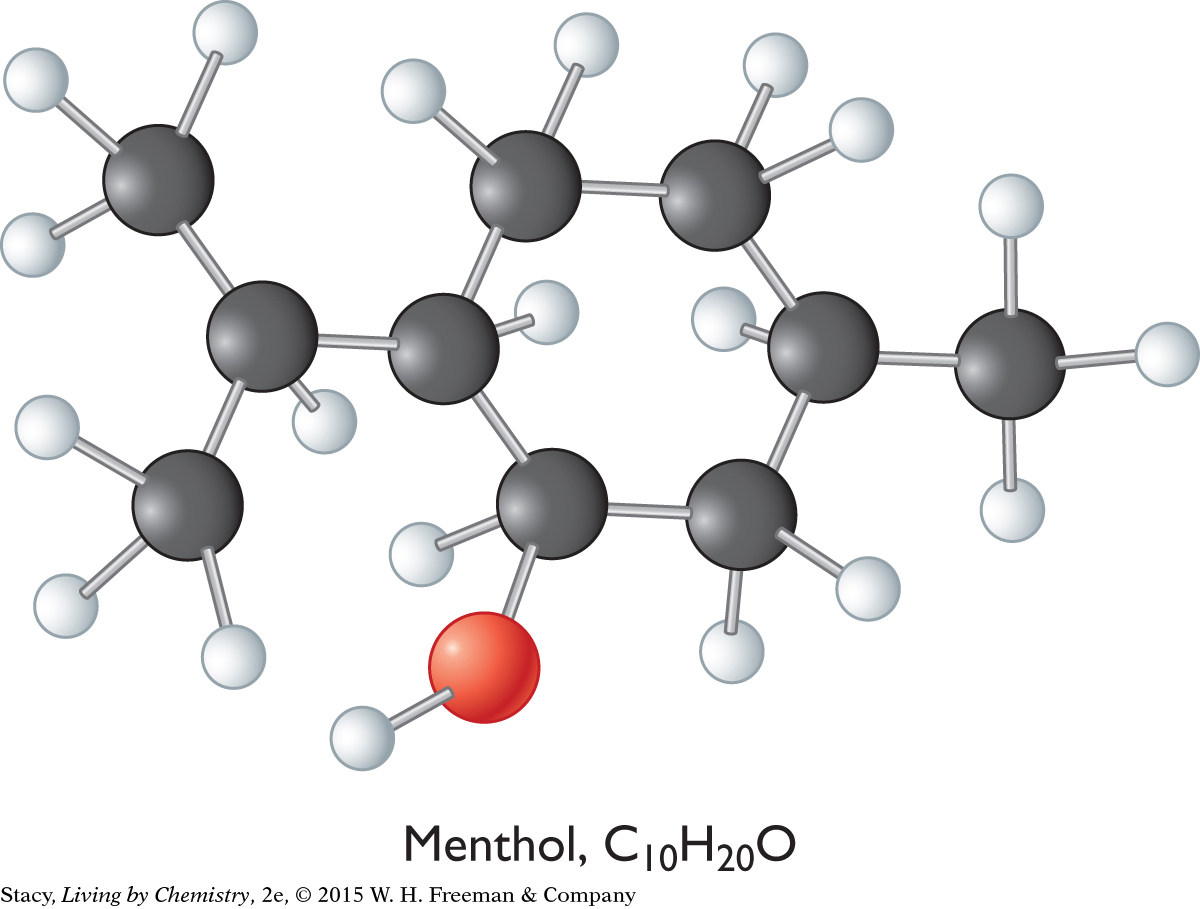
Ball-and-stick models for the three compounds introduced at the beginning of this lesson are below.
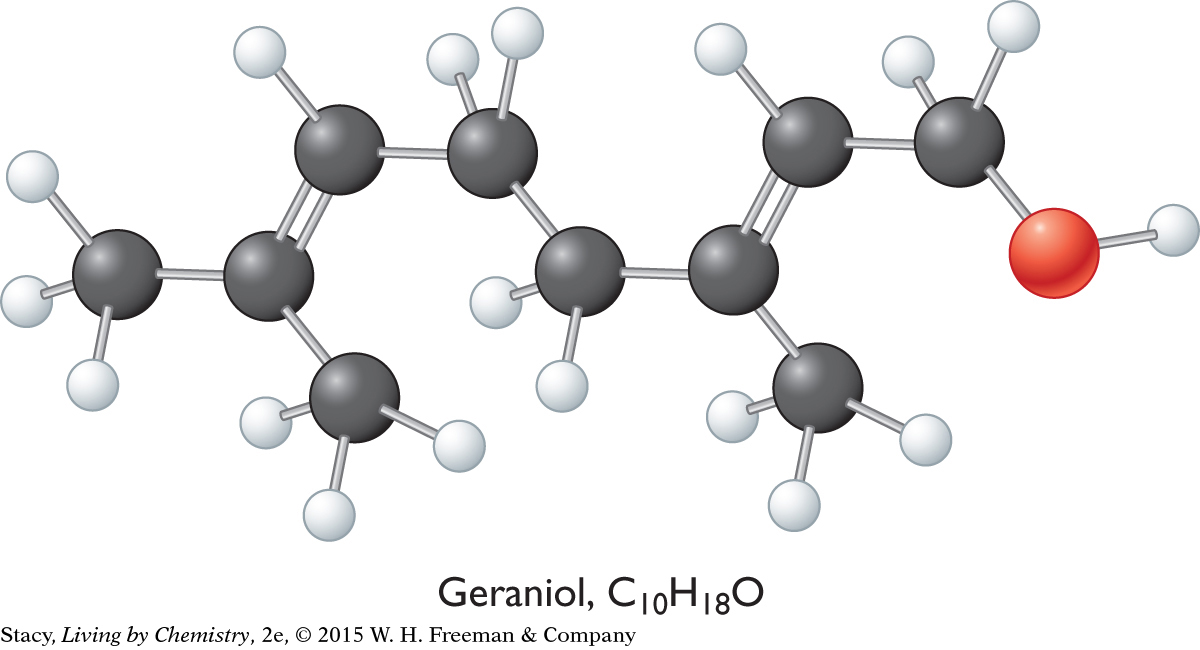
Geraniol, C10H18O
|
Fenchol, C10H18O
|
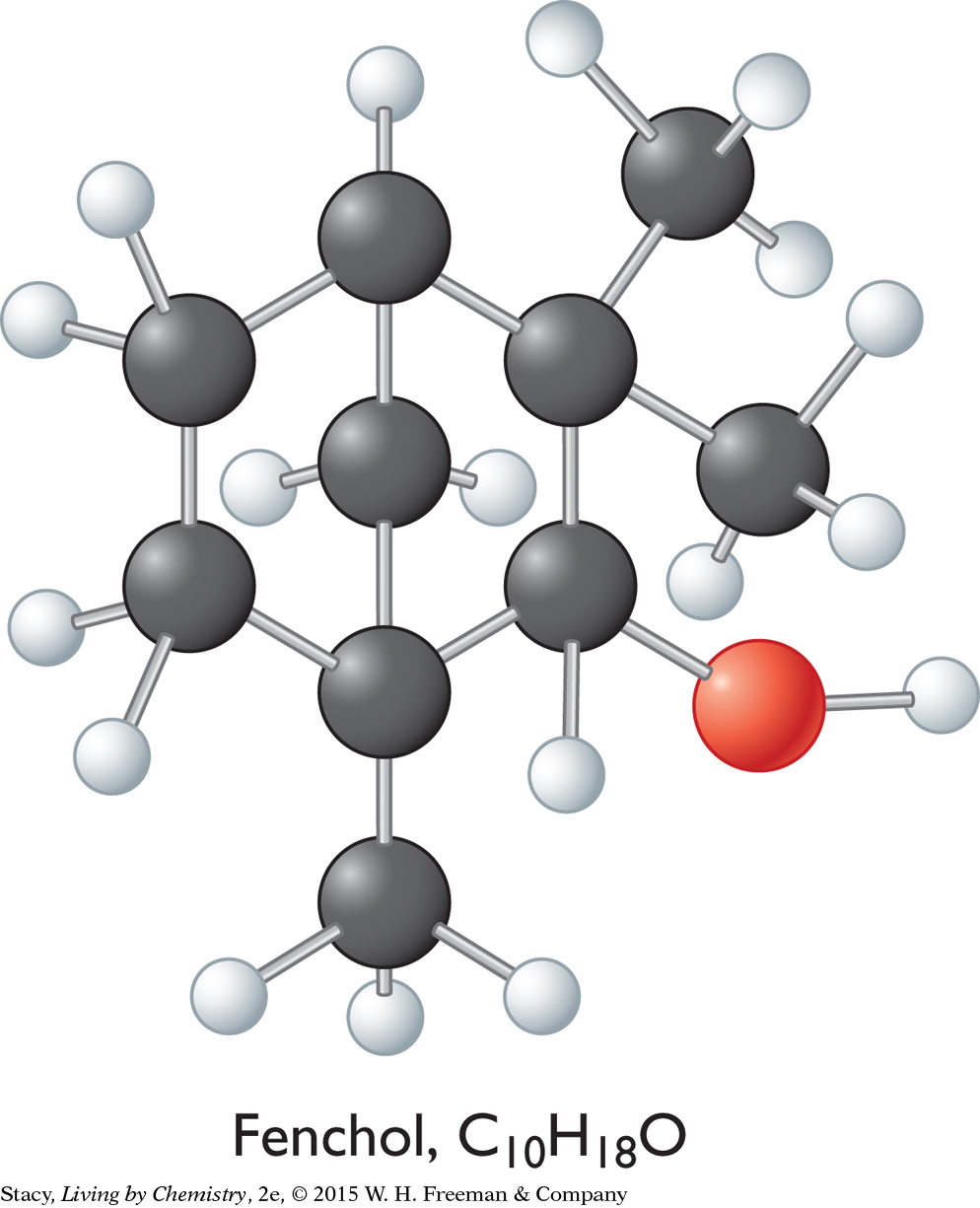
The overall shapes of the molecules differ greatly. The carbon atoms in geraniol are connected in a long chain, while the menthol and fenchol molecules both contain ring structures. Perhaps these overall shapes are related to the different smells of these three alcohols.
LESSON SUMMARY
LESSON SUMMARY
What three-dimensional features of a molecule are important in predicting smell?
KEY TERM
ball-and-stick model
Molecules are three-dimensional. A ball-and-stick model kit is a tool that you can use to construct models of molecules. Ball-and-stick models allow you to see how the various atoms in molecules are arranged in space, as well as the overall shape of each molecule. Molecular shape may have something to do with smell.
Exercises
Reading Questions
What are the differences between a structural formula and a ball-and-stick model?
What is your hypothesis about why the three alcohols smell different even though they have the same functional group?
Reason and Apply
189
What model pieces do you need to build a ball-and-stick model of geraniol?
Consider this model. Its molecular formula is C7H14O2.
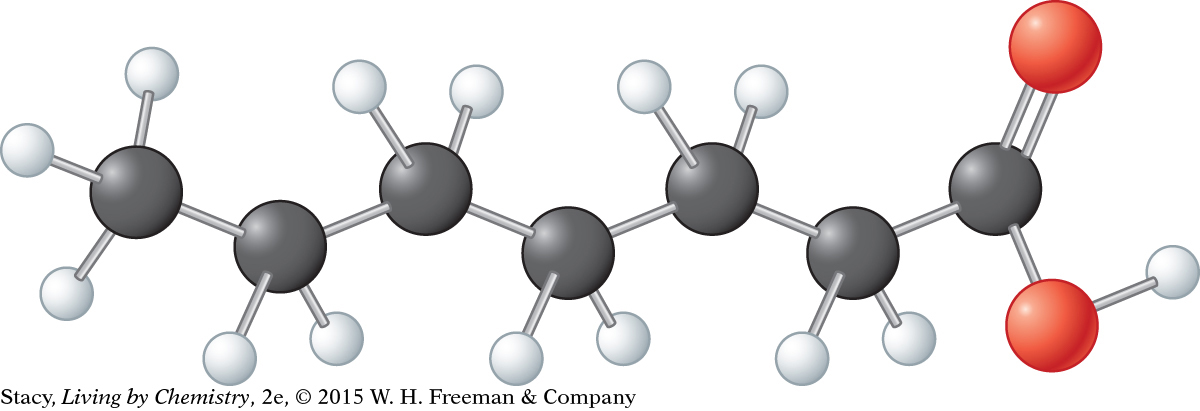
Draw the Lewis dot structure.
Draw the structural formula.
What is the functional group in the molecule?
What can you predict about the name and smell of this compound?
Consider this model. Its molecular formula is C3H6O2.
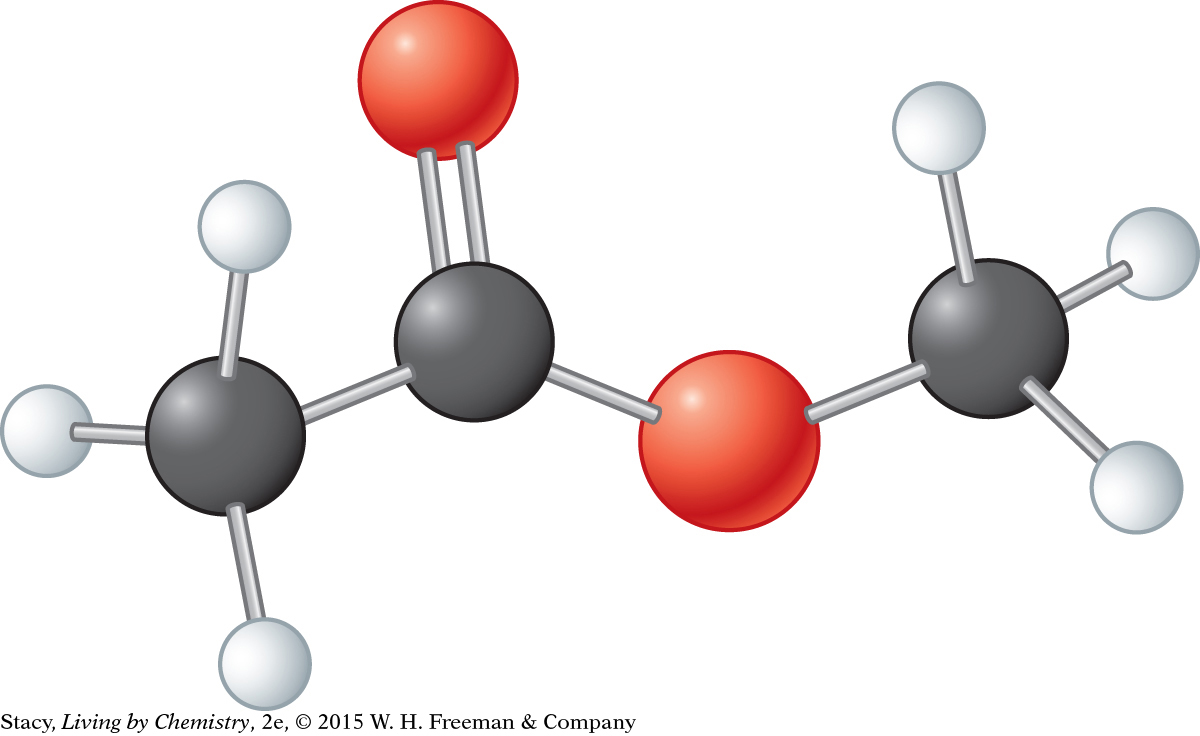
Draw the Lewis dot structure.
Draw the structural formula.
What is the functional group in the molecule?
What can you predict about the name and smell of this compound?
Consider this model. Its molecular formula is C2H7N.
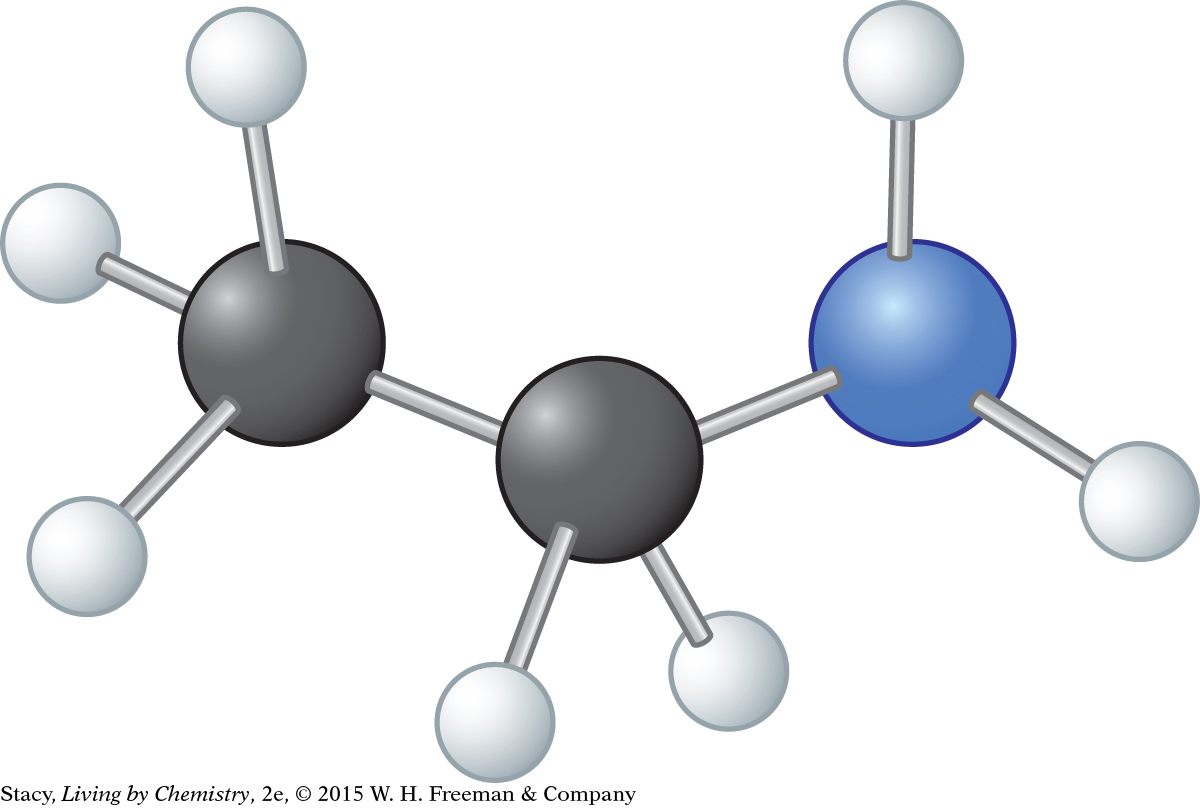
Draw the Lewis dot structure.
Draw the structural formula.
What is the functional group in the molecule?
What can you predict about the name and smell of this compound?
What evidence do you have that the structural formula may not always be useful in predicting smell?