LESSON 37: Two’s Company: Electron Domains
191
THINK ABOUT IT
You may have noticed that the atoms in the molecular model kits have a certain number of holes and that the sticks only fit in certain places. So, when you connect the atoms, the molecules automatically end up with the correct three-dimensional shape. But what is it that determines the shape of a molecule?
How do electrons affect the shape of a molecule?
To answer this question, you will explore
Shapes of Molecules
Electron Domains
Shapes of Molecules
EXPLORING THE TOPIC
Shapes of Molecules
These illustrations show models of ethanol, C2H6O.
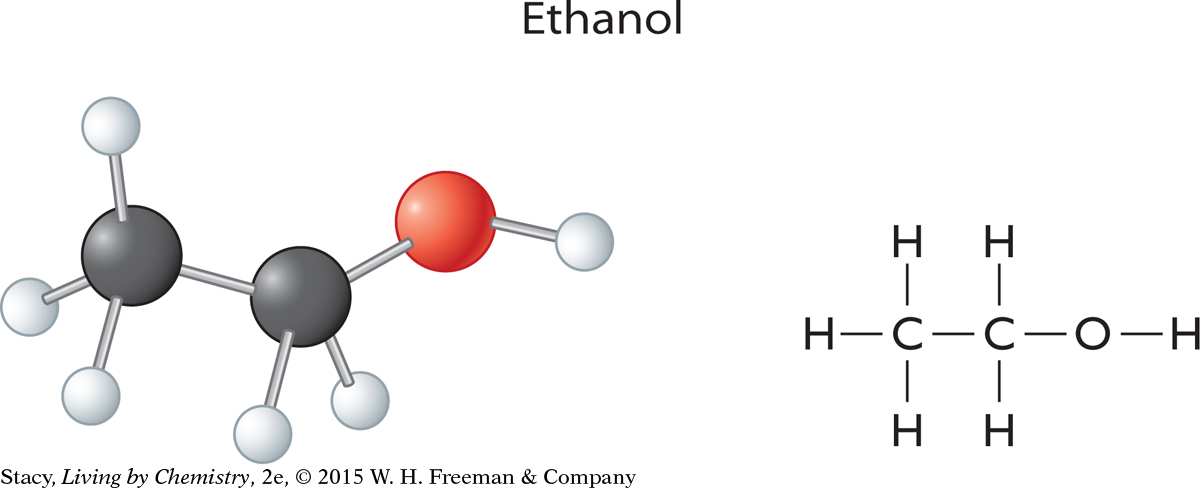
What accounts for the three-dimensional structure of ethanol? The answer lies in the valence electrons. They determine the ultimate shape of a molecule.
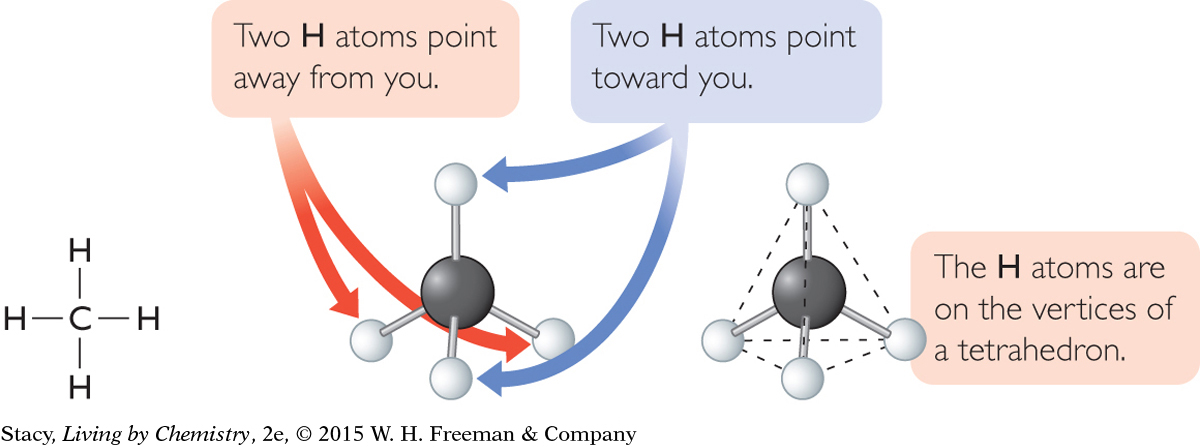
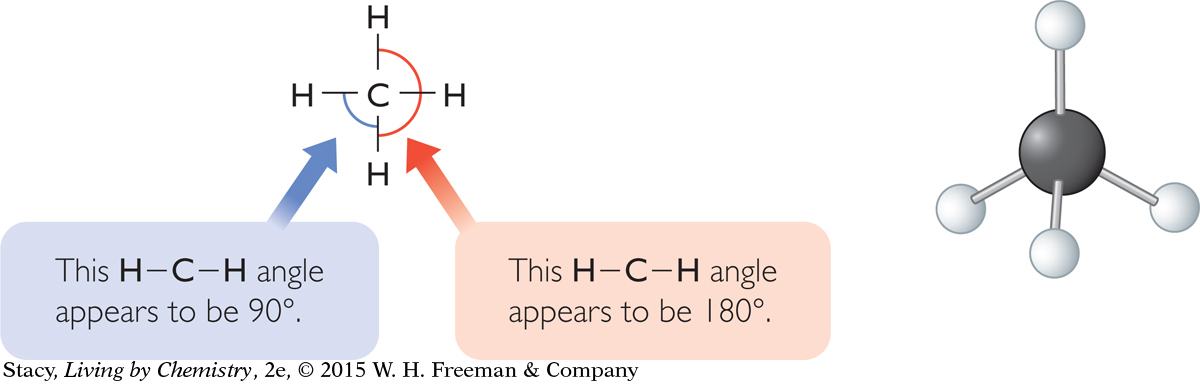
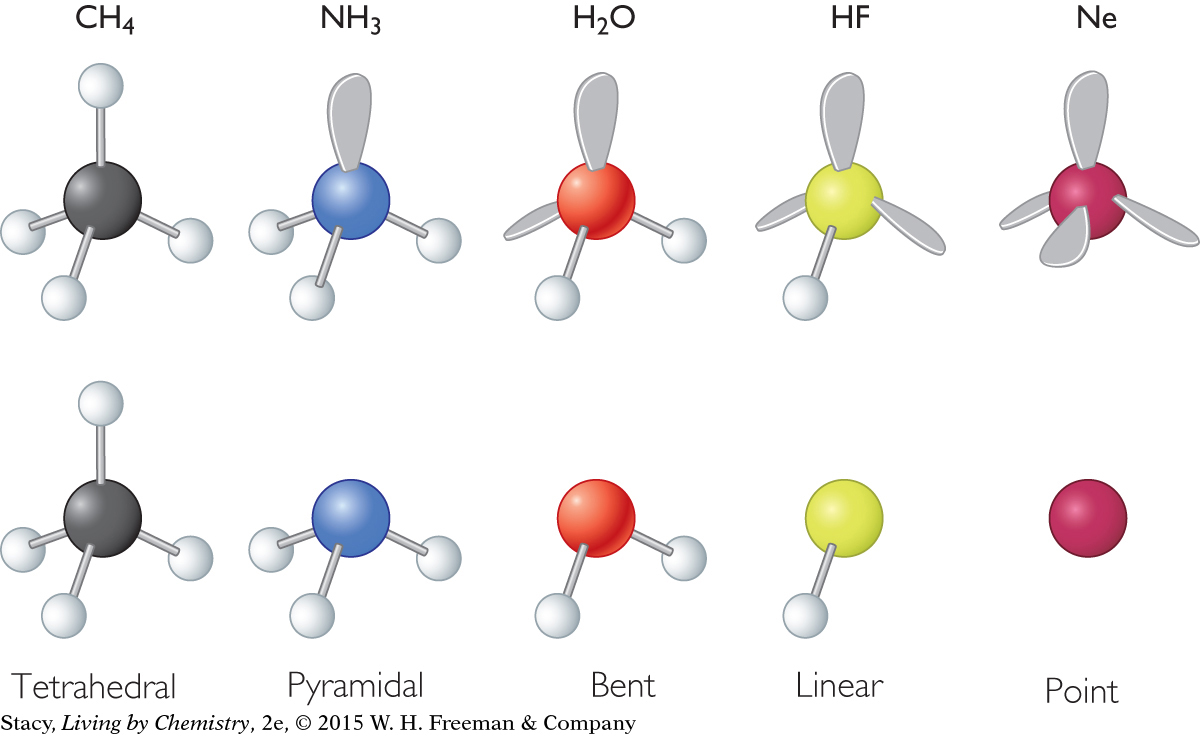
To understand molecular shape, it is useful to begin by examining a simple molecule such as methane, CH4. While the structural formula is flat and cross-shaped, the ball-and-stick model shows that methane has a three-dimensional structure. Both models show that there are four bonds.
192
The word used to describe the shape of the methane molecule is tetrahedral. A tetrahedral molecule has a symmetrical shape, with one atom exactly in the center. The distance between any other two atoms bonded to the central atom is the same.
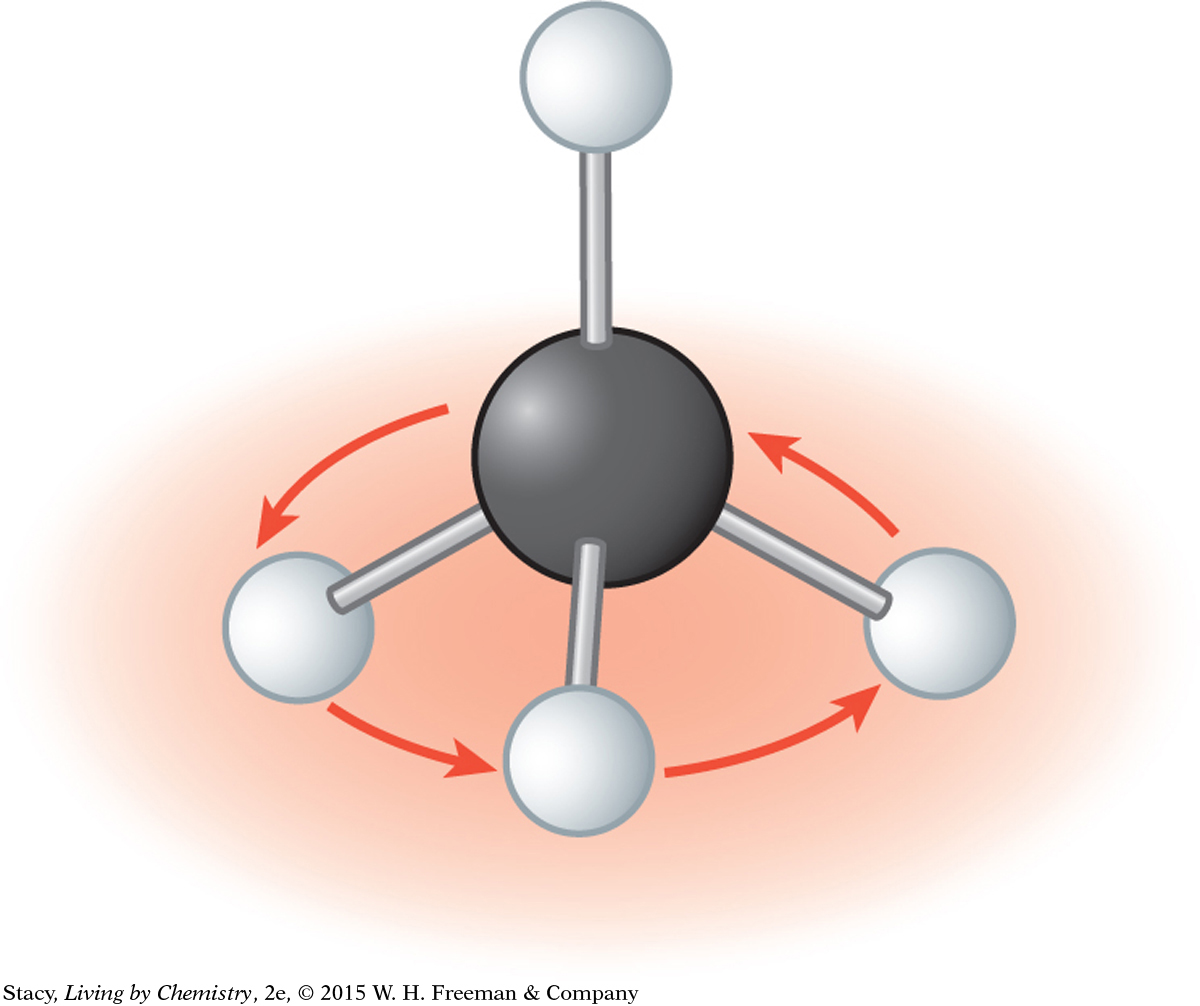
You can prove to yourself that a ball-and-stick model of methane is symmetrical in every direction by spinning the molecule as shown in the illustrations. The molecule looks exactly the same no matter what direction it is spun.
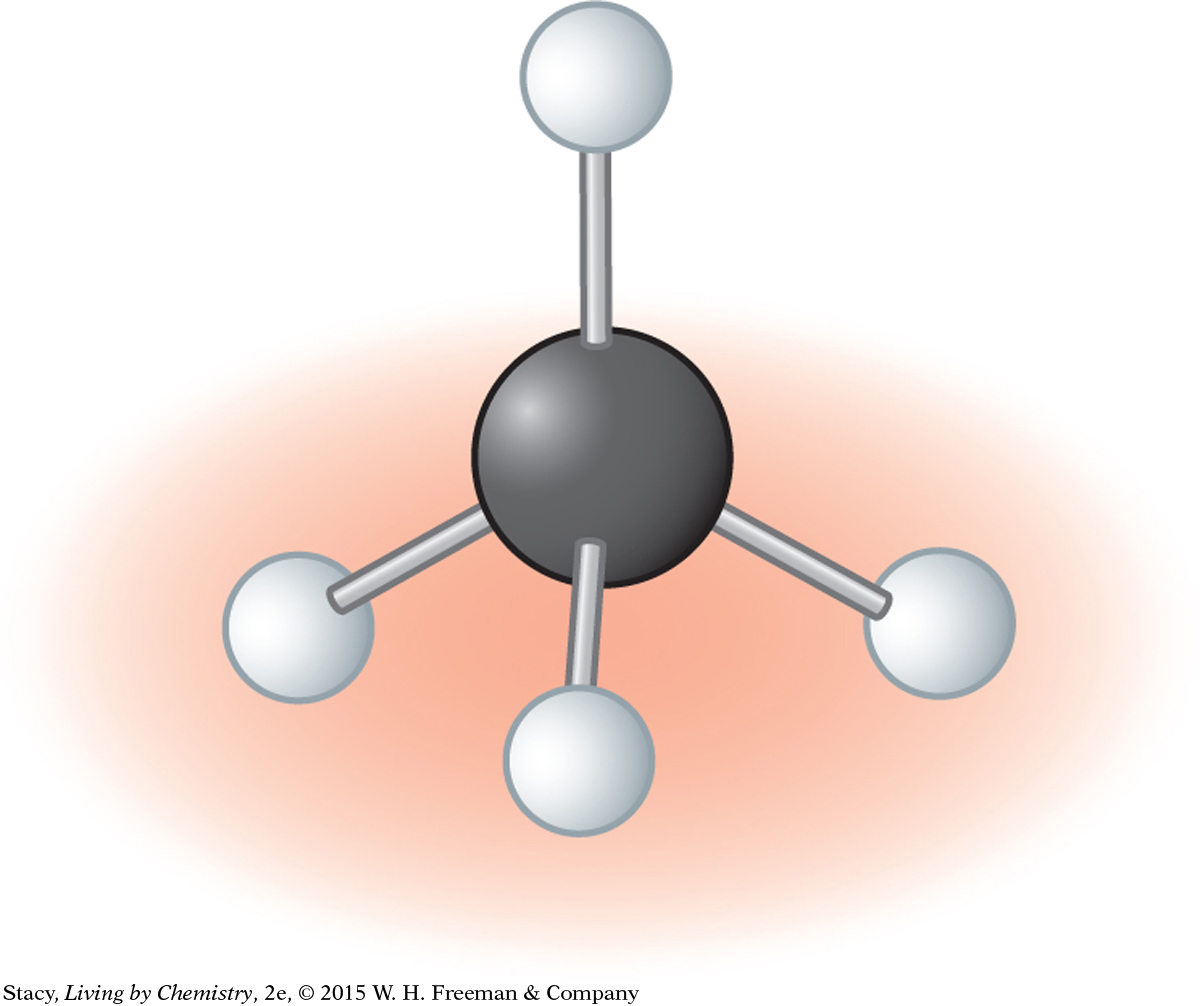
One H atom sticks straight up. Three H atoms rest on the table.
|
You can spin the molecule around and it looks identical.
|
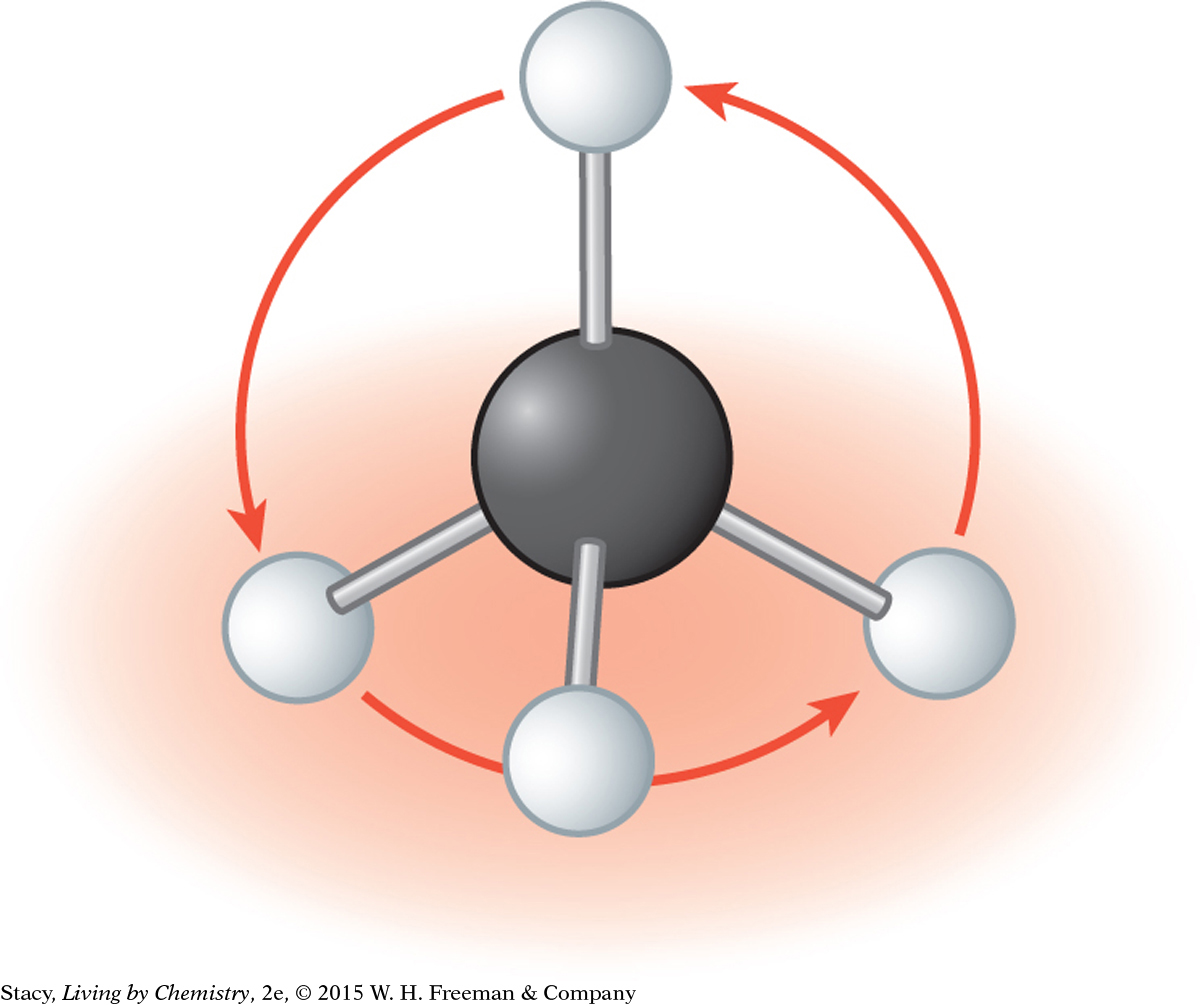
You can grab one of the H atoms on the table and spin the molecule around and it still looks identical.
|
Electron Domains
Electron Domains
METHANE, CH4
The illustration here shows a methane molecule with the shared valence electrons superimposed, or laid, over the ball-and-stick model. The bonded electron pairs occupy space between the carbon atom and each of the four hydrogen atoms. The space occupied by the electrons is called an electron domain. An electron domain can consist of a bonded pair of electrons, a lone pair of electrons, or multiple bonded pairs of electrons.
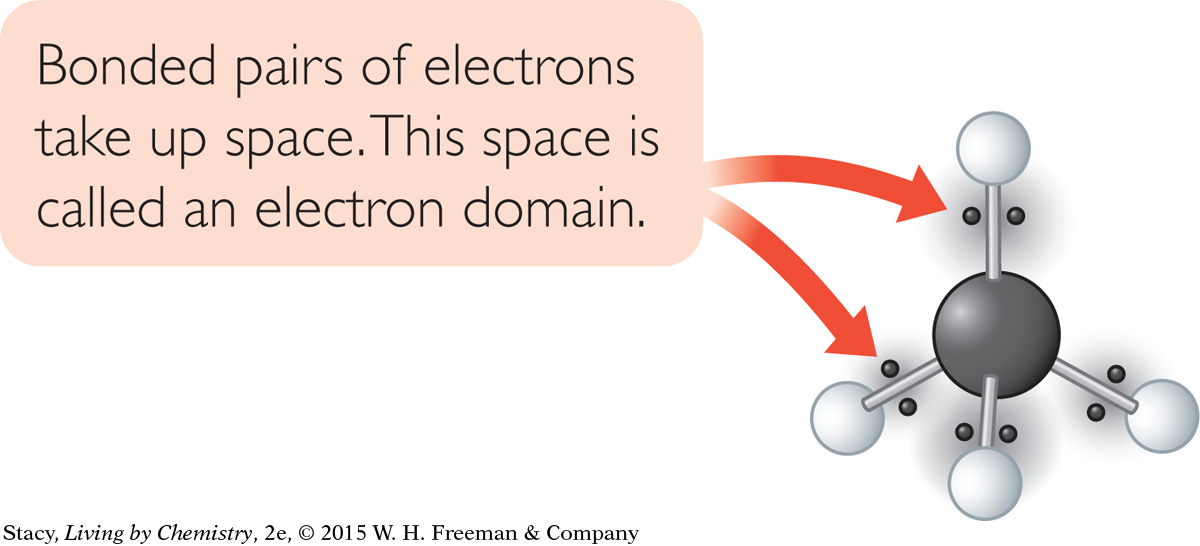
193
Each electron domain in the tetrahedral methane molecule is the same distance from the other three electron domains. They are as far apart from one another as possible. Because of this arrangement, the hydrogen atoms are as far apart from one another as possible and the distance between any two hydrogen atoms is the same. Additionally, in the tetrahedral molecule, if you measure the angle formed between any two hydrogen atoms and the carbon atom, you’ll find that all these bond angles have equal measures. This is not accurately shown in the structural formula.
CONSUMER CONNECTION

CONSUMER
CONNECTION
Molecules containing only carbon and hydrogen atoms are generally referred to as hydrocarbons. The simplest hydrocarbon compound is methane. Notice that methane’s name ends in “-ane” because it is an alkane. Many of the medium-sized alkane molecules smell like octane, a major component of gasoline.
Important to Know
Electrons are negatively charged, so they repel one another. However, in molecules, electrons bond in pairs. It is the electron pairs that repel one another.
This tendency for electron pairs to be as far apart from one another as possible is called electron domain theory. This theory is also referred to as valence shell electron pair repulsion theory, or VSEPR theory.
AMMONIA, NH3
The structural formula and ball-and-stick model for ammonia, NH3, are shown below. The ball-and-stick model shows that the hydrogen atoms are all located on one side of the nitrogen atom.
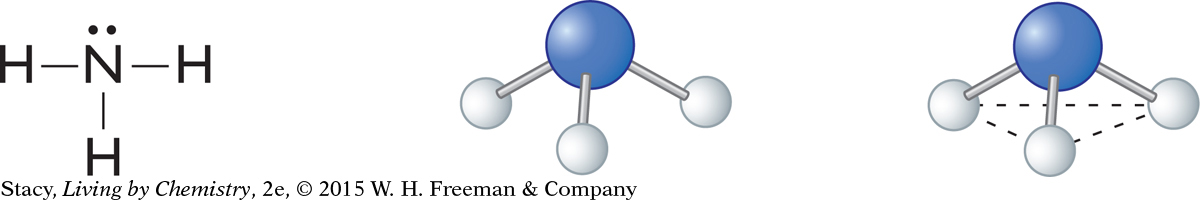
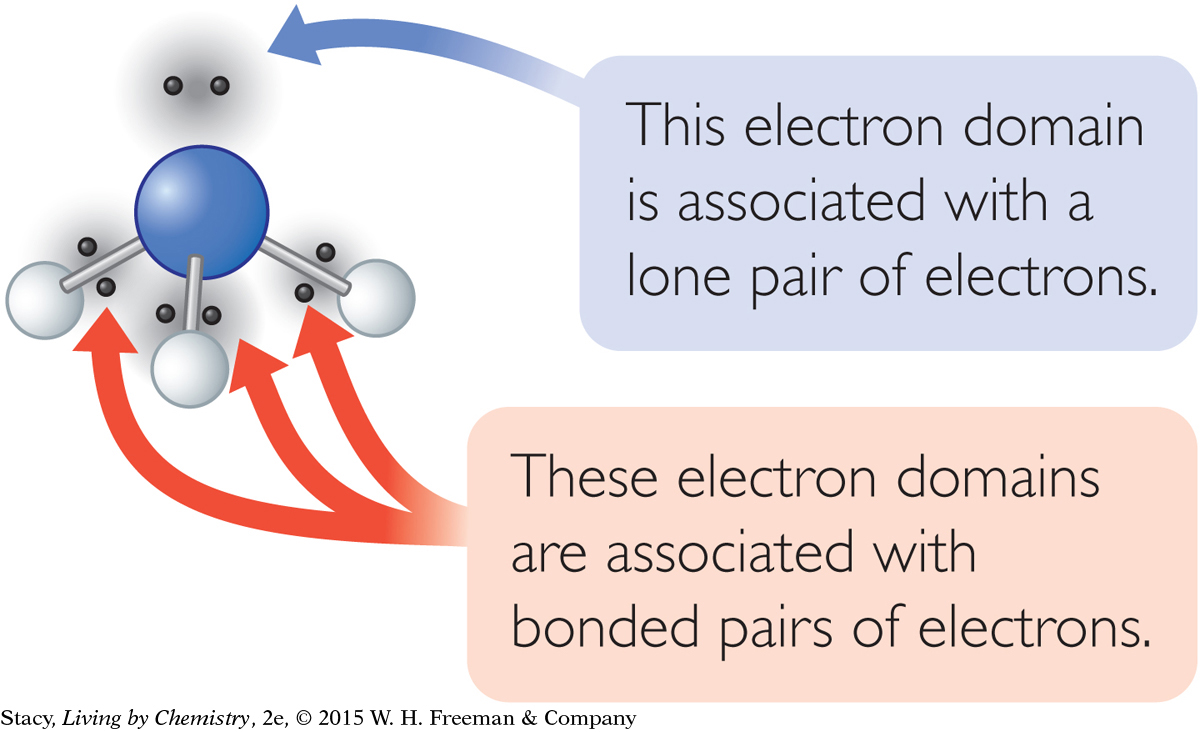
The word used to describe the shape of the ammonia molecule is pyramidal.
The nitrogen atom has a lone pair of electrons. Therefore, while there are only three bonds, there are four electron domains. The four electron domains are arranged in a tetrahedral shape to get as far apart as possible, similar to what happens in methane.
WATER, H2O
194
Take a look at one more molecule—water, H2O. The word used to describe the shape of a water molecule is bent.
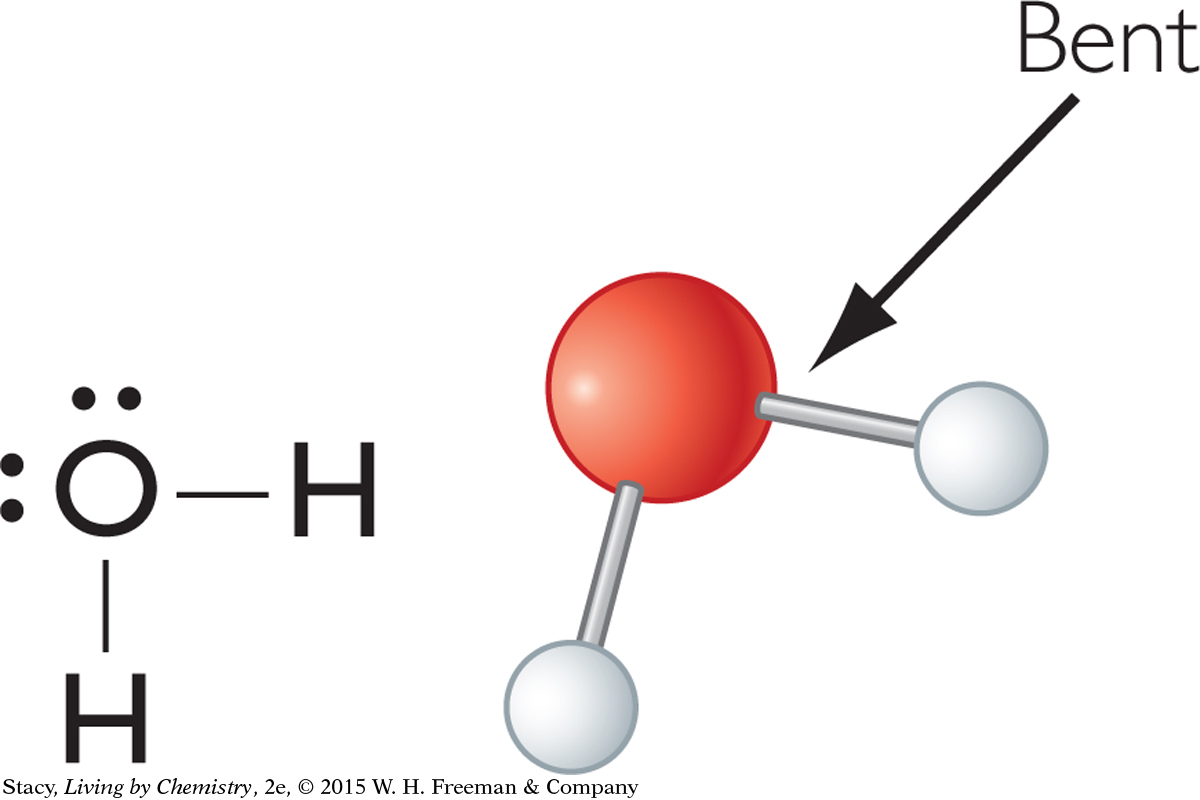
A water molecule has four electron domains, two bonding pairs and two lone pairs. The four electron domains of water are arranged in a tetrahedral shape, as in methane and ammonia. This results in a bent shape for the water molecule. In all three molecules, the bond angles are 109º or close to this.
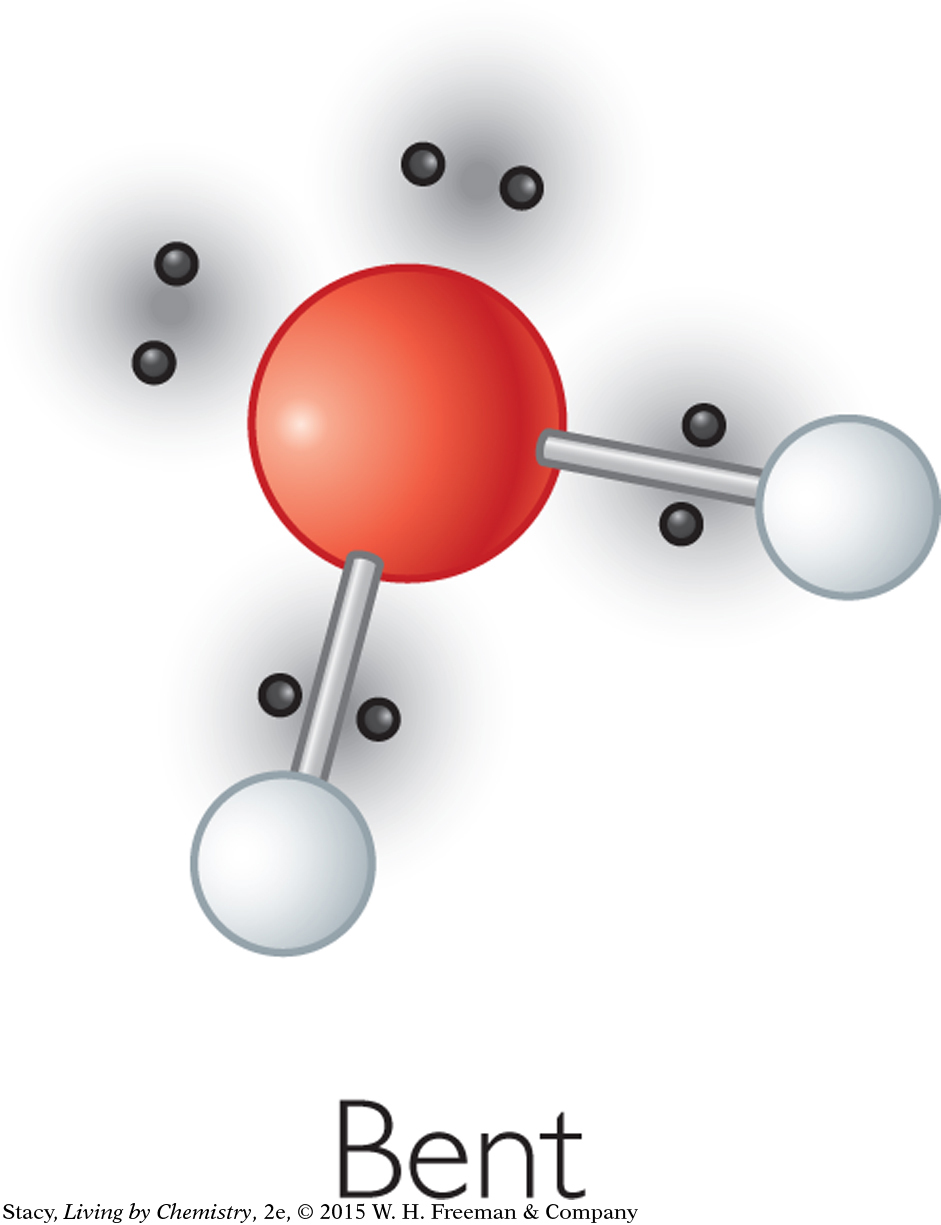
BALL-AND-STICK MODELS WITH LONE PAIR PADDLES
In a molecular model kit, the lone pairs are sometimes represented as plastic paddles. This helps you to visualize where the final shape of a molecule comes from.
AGRICULTURAL CONNECTION
AGRICULTURAL
CONNECTION
Phosphine is a highly toxic, highly flammable, colorless gas used as a fumigant to kill insects that might damage stored grain.

Notice that you name the shape of the molecule based only on the arrangement of atoms. The lone pairs are not considered.
195
Example
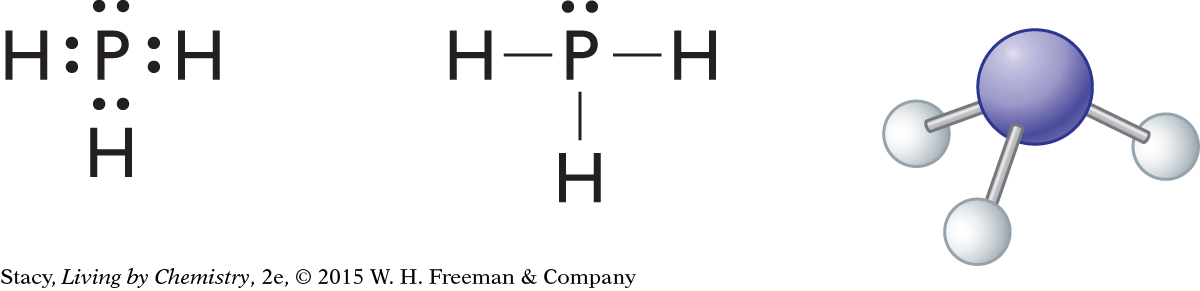
Phosphine, PH3
What is the shape of phosphine, PH3? Explain your thinking.
Solution
Begin by drawing the Lewis dot structure and a structural formula of phosphine. A phosphorus atom has five valence electrons, and each hydrogen atom contributes one electron, for a total of eight electrons. There are three bonding pairs and one lone pair. The four atoms in phosphine are arranged in a pyramidal shape.
LESSON SUMMARY
LESSON SUMMARY
How do electrons affect the shape of a molecule?
KEY TERMS
tetrahedral shape
electron domain
electron domain theory
pyramidal shape
bent shape
The three-dimensional shape of a molecule is determined by the various electron pairs in the molecule. Both bonded pairs of electrons and lone pairs of electrons affect the shape of a molecule. Each electron pair takes up space, called an electron domain. The final shape of a molecule is determined by the fact that electron domains in a molecule are located as far apart from one another as possible.
Exercises
Reading Questions
In your own words, describe a tetrahedral shape.
What is meant by electron domain theory?
Reason and Apply
If you were going to predict the three-dimensional structure of a small molecule, what would you want to know?
Predict the three-dimensional structure of H2S. Explain your thinking.
List three molecules that have a tetrahedral shape.
List three molecules that have a bent shape.
What is the shape of arsine, AsH3? Explain your thinking.
Predict the three-dimensional shape of HOCl. Explain your thinking.
Draw a methane, CH4, molecule and show how it fits inside a tetrahedron. Do the same for ammonia, NH3, and water, H2O.