LESSON 38: Let’s Build It: Molecular Shape
196
THINK ABOUT IT
So far you have considered the shapes of small molecules containing single bonds. All of the molecules you’ve studied have had four electron domains. However, small molecules that have only three or even two electron domains also exist. These molecules have double and triple bonds. So, there are more molecular shapes to consider.
How can you predict the shape of a molecule?
To answer this question, you will explore
More Shapes
Larger Molecules
More Shapes
EXPLORING THE TOPIC
More Shapes
DOUBLE BONDS
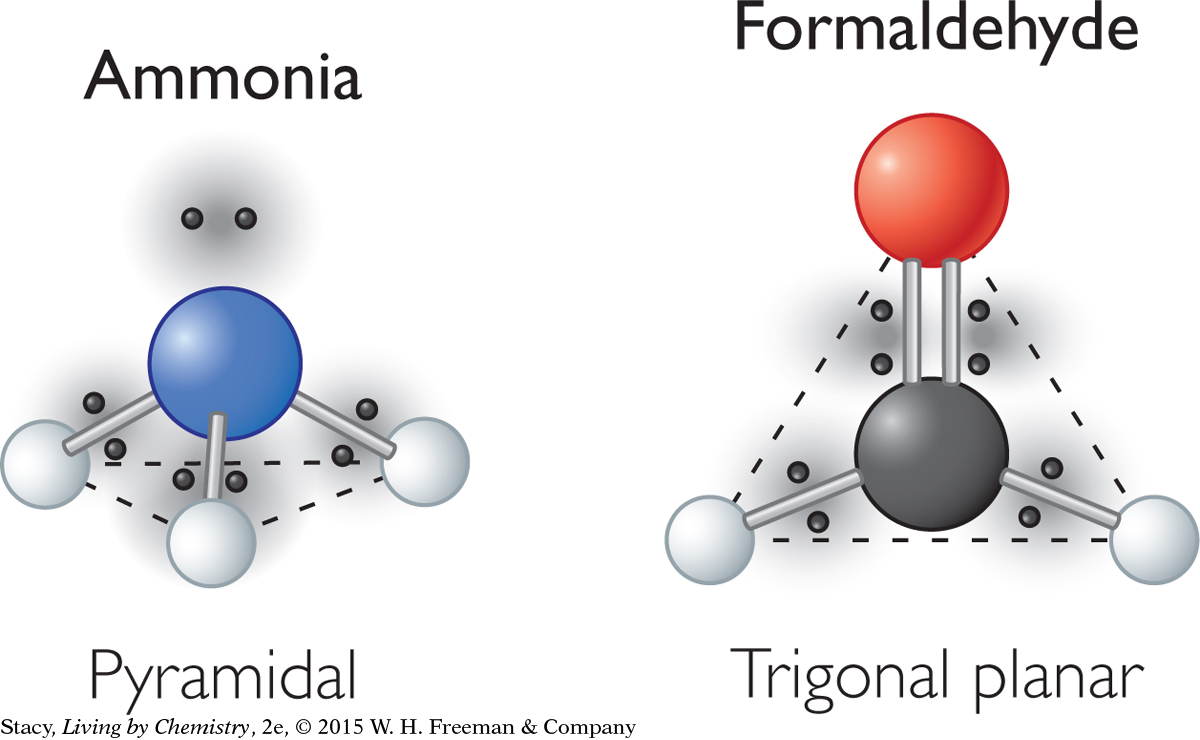
Formaldehyde, CH2O, is a very simple molecule that contains one double bond. The double bond counts as only one electron domain even though it contains two pairs of bonded electrons. This means that the central atom in the formaldehyde molecule (the carbon atom) has only three electron domains surrounding it, spread out into a flat triangle. The phrase used to describe the underlying shape of the formaldehyde molecule is trigonal planar.
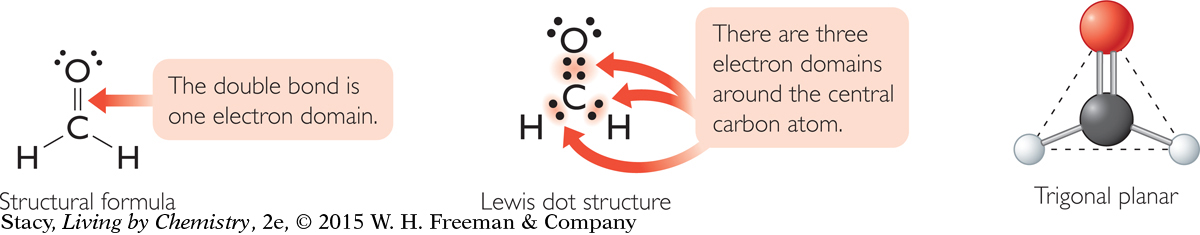
Both ammonia, NH3, and formaldehyde, CH2O, have four atoms but have very different shapes. The difference in shape is due to the number of electron domains around the central atom.
TWO DOUBLE BONDS
197
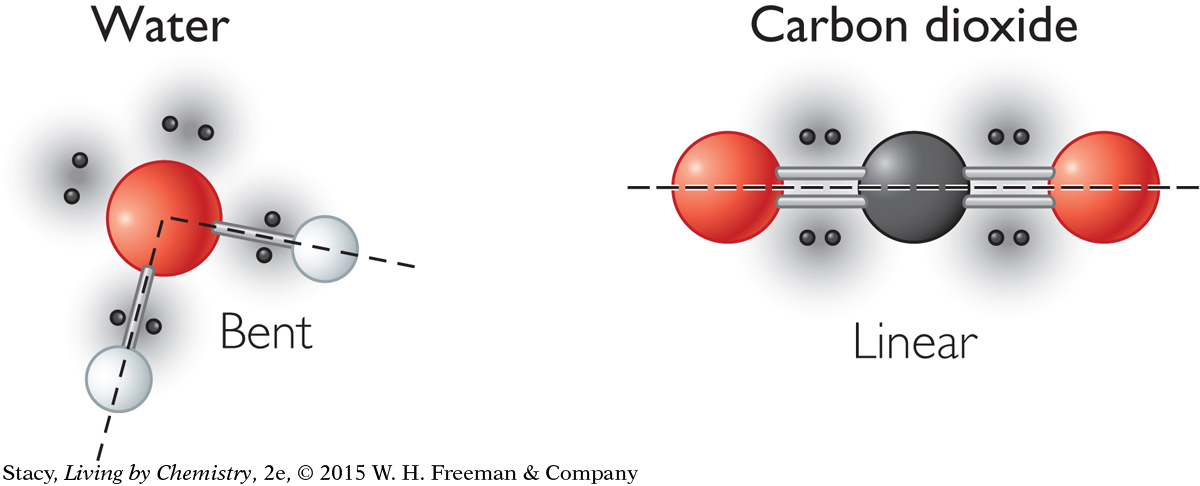
In a molecule with a double bond, the double bond counts as one electron domain. So a molecule like carbon dioxide, with two double bonds, has only two electron domains around the central atom that have to be as far apart as possible. This shape is described as linear.
Important to Know
The four electrons in a double bond remain between two atoms, so they are considered one electron domain. An electron domain can have two, four, or six electrons.
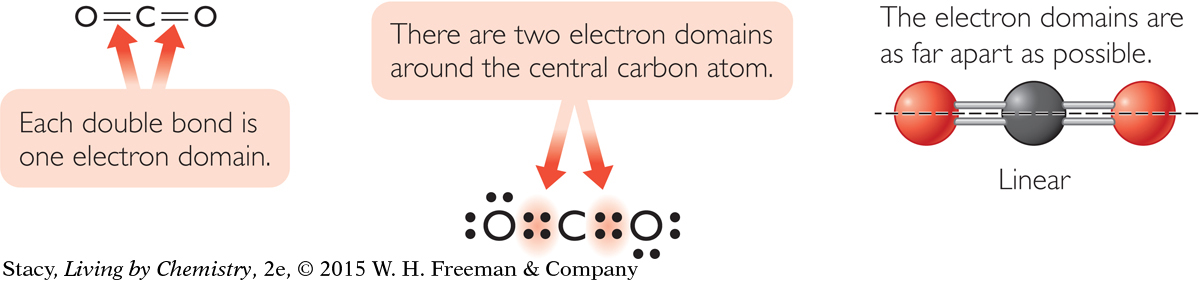
Both water, H2O, and carbon dioxide, CO2, have three atoms. However, the shape of water is bent while carbon dioxide is linear. The difference in shape is due to the number of electron domains around the central atom.
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Ethene, C2H4, is also called ethylene. It is a colorless gas with a slightly sweet odor. Ethene is used in the manufacture of many common products like polyethylene plastic and polystyrene. Ethene also can be used as a plant hormone to control the ripening of fruit or the opening of flowers.

Example 1
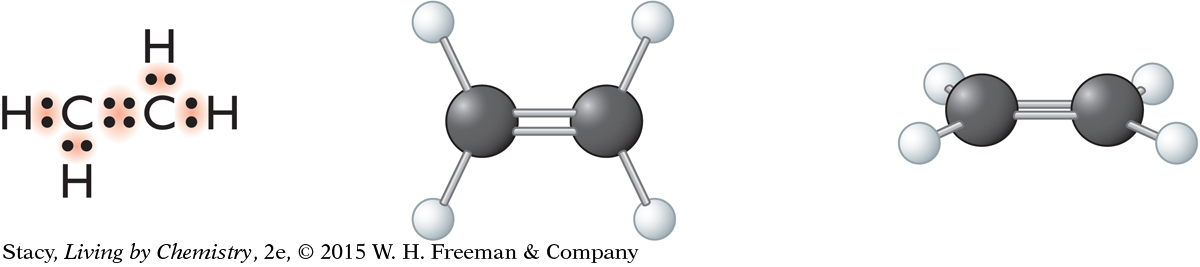
Ethene, C2H4
Determine the molecular shape of ethene, C2H4.
Solution
Begin by drawing the Lewis dot structure for ethene to determine the number of electron domains around each carbon atom. Next, translate that into a three-dimensional representation of the molecule. In this case, there are no lone pairs. The molecule is flat. Each half of ethene is trigonal planar.
TRIPLE BONDS
198
Another example of a linear molecule is hydrogen cyanide, HCN. It also has two electron domains around the central atom, like carbon dioxide. One electron domain is a single bond between carbon and hydrogen, and the other is a triple bond between carbon and nitrogen.

Example 2
Ethyne, C2H2
Determine the molecular shape of ethyne, C2H2.
Solution
Begin by drawing the Lewis dot structure of ethyne to determine the number of electron domains around the carbon atoms. To satisfy the octet rule and the HONC 1234 rule, the carbon atoms must have a triple bond between them. Each carbon atom has two electron domains surrounding it. Ethyne is a linear molecule.

Larger Molecules
Larger Molecules
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Ethyne, C2H2, is also called acetylene. It is the highly flammable gas used by welders in oxyacetylene torches. Miners’ helmets used to have carbide lamps attached to them. A carbide lamp is based on the reaction between calcium carbide, CaC2, and water, H2O, to produce acetylene, C2H2. The acetylene gas burns to produce a bright light in the dark cave.

The shapes of all of these small molecules help to explain why chains of carbon atoms are crooked rather than straight. The illustration shows a ball-and-stick model for heptanoic acid, C7H14O2, with seven carbon atoms in a chain. The arrangement of atoms around six of the carbon atoms is tetrahedral. Most of the molecule is a series of overlapping tetrahedral shapes.
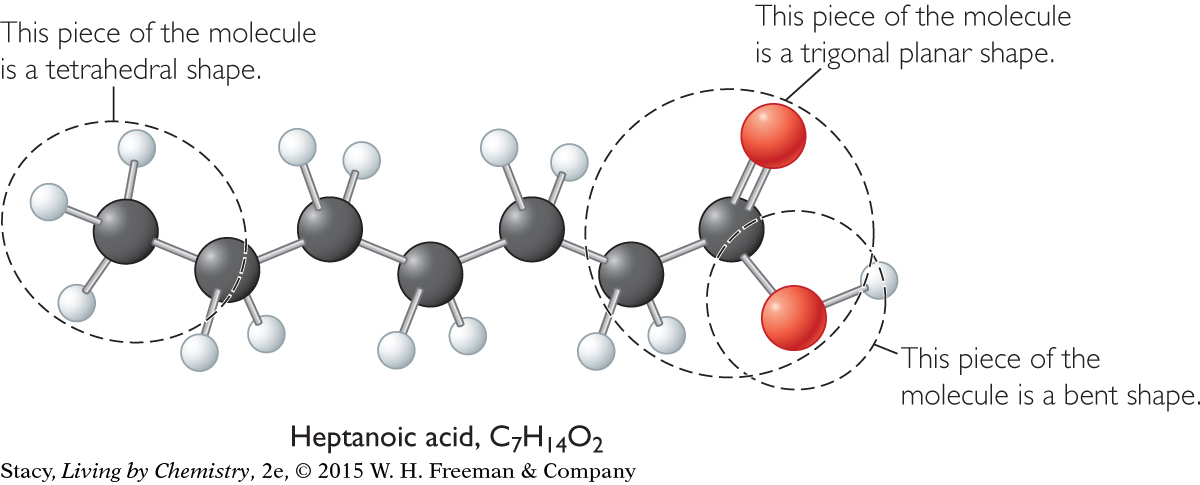
The various electron domains in this molecule cause its overall shape to be crooked, or zigzag.
LESSON SUMMARY
LESSON SUMMARY
199
KEY TERMS
trigonal planar shape
linear shape
How can you predict the shape of a molecule?
The shape of a molecule is affected by the location and number of its electron domains. Lewis dot structures help you to determine the number of electron domains in a molecule. An atom involved in a double bond or triple bond will have fewer electron domains surrounding it. This results in molecules that have trigonal planar and linear shapes. Large molecules can have various geometric shapes within them. Areas around double bonds are flat, and those around triple bonds are linear.
Exercises
Reading Questions
What shapes are possible if a molecule has three electron domains?
What shapes are possible for a molecule with three atoms? Explain your thinking.
Reason and Apply
Describe the shape of each of these molecules. (Use Lewis dot structures, the periodic table, and the HONC 1234 rule to assist you.)
Cl2 CO2 H2O Which of the molecules in Exercise 3 have the same shape?
What shape or shapes do you predict for a molecule with two atoms? A molecule with three atoms? Four atoms? Five atoms? You can draw Lewis dot structures or electron domains to assist you in answering the questions.
Predict the shapes of these molecules:
CF4 NF3 H2Se H2CS Consider the butane, C4H10, molecule.
Draw the Lewis dot structure for butane.
How many electron domains does the molecule have?
What shape would you predict for C4H10?
Explain why the carbon atom chain is not straight.