LESSON 39: What Shape Is That Smell?: Space-Filling Models
THINK ABOUT IT
200

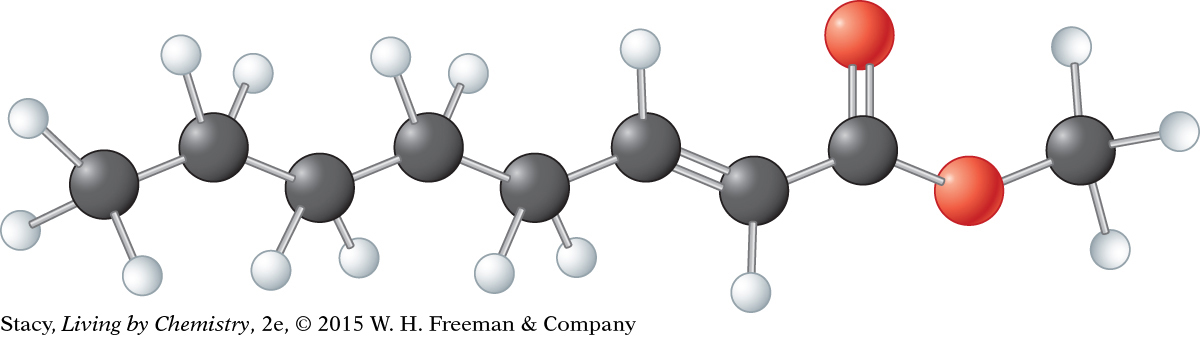
Methyl octenoate, C9H16O2, is a compound that smells like violets. A ball-and-stick model of methyl octenoate shows that it is a series of overlapping tetrahedral shapes stuck together. There is a trigonal planar segment in the area of the double bonds. But how would you describe the shape of the whole molecule? And does the shape of the whole molecule have anything to do with its smell?
How is the shape of a molecular compound related to its smell?
To answer this question, you will examine
Space-Filling Models
Relating Shape and Smell
Space-Filling Models
EXPLORING THE TOPIC
Space-Filling Models
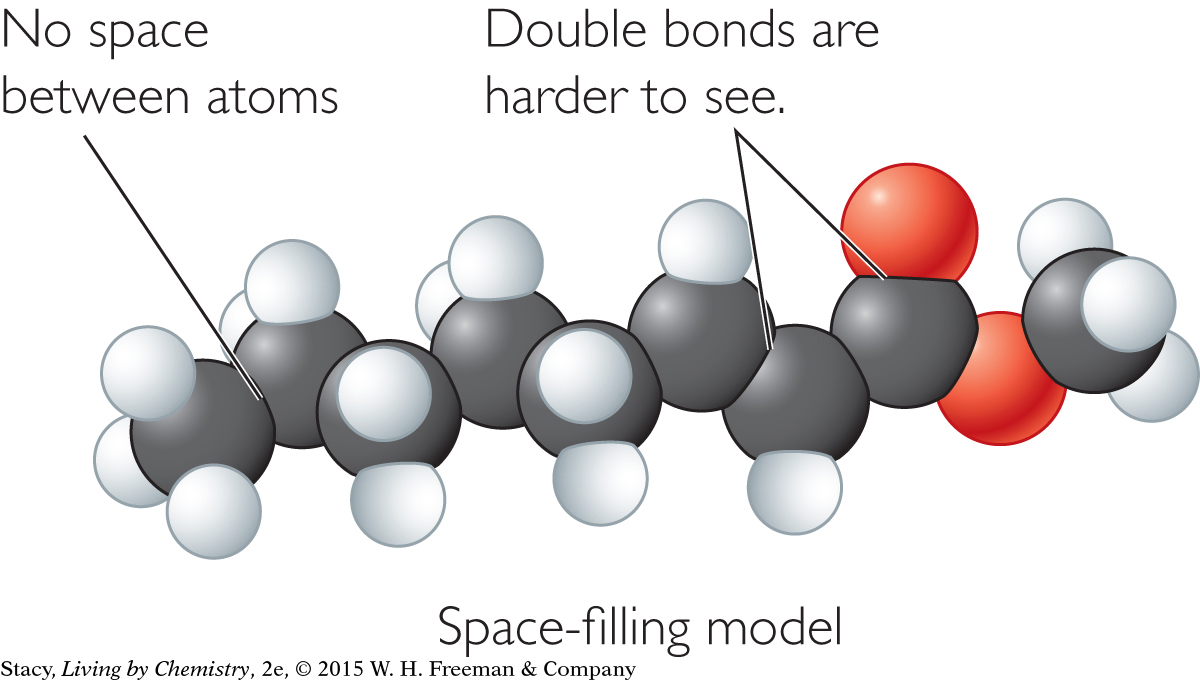
Most of the smell molecules you have encountered are considerably larger than three, four, or five atoms. The best way to look at the overall shape of these molecules is with a different type of model, called a space-filling model.
Take a moment to compare the ball-and-stick model of methyl octenoate with the space-filling model. In a space-filling model, the sticks between atoms have been eliminated. There is no space between atoms. Instead, bonded atoms are shown slightly overlapping.
Methyl octenoate, C9H16O2
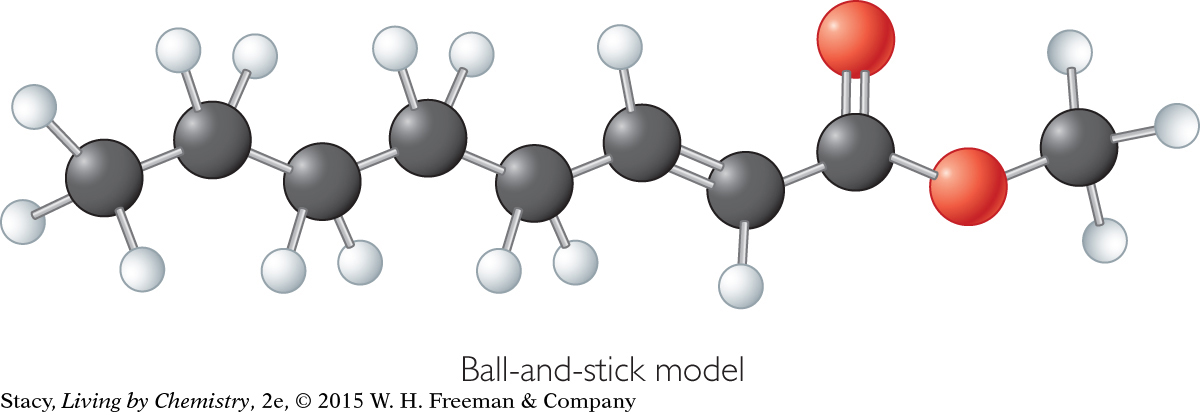
Ball-and-stick model
|
space-filling model
|
201
In some ways, a space-filling model could be considered more accurate than a ball-and-stick model. In reality, a stick has no resemblance to a bond. In a molecule, the atoms share electrons with neighboring atoms. This would suggest that the atoms are located very close to, or overlapping, one another. However, in an illustration of a space-filling model, you can’t see multiple bonds, and some atoms may be hidden behind others.
Relating Shape and Smell
Relating Shape and Smell
Space-filling models for molecules that smell sweet, minty, and like camphor (piney) are shown here. Take a moment to look for patterns that may indicate a connection between the shapes of these molecules and their smells.
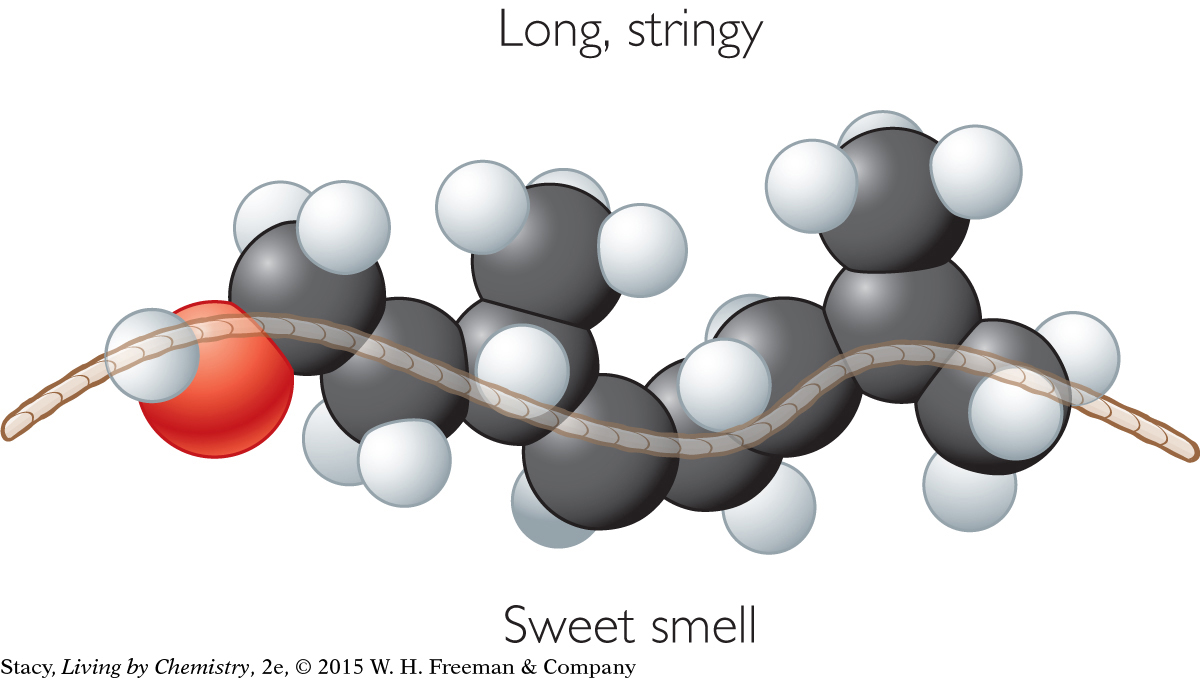
SWEET-SMELLING MOLECULES
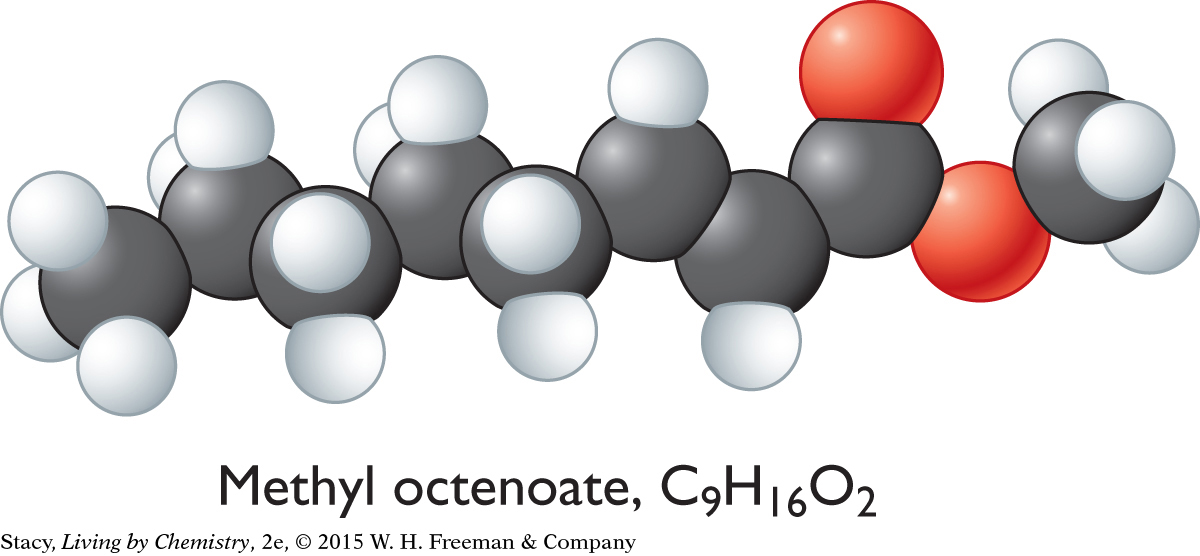
Methyl octenoate, C9H16O2
|
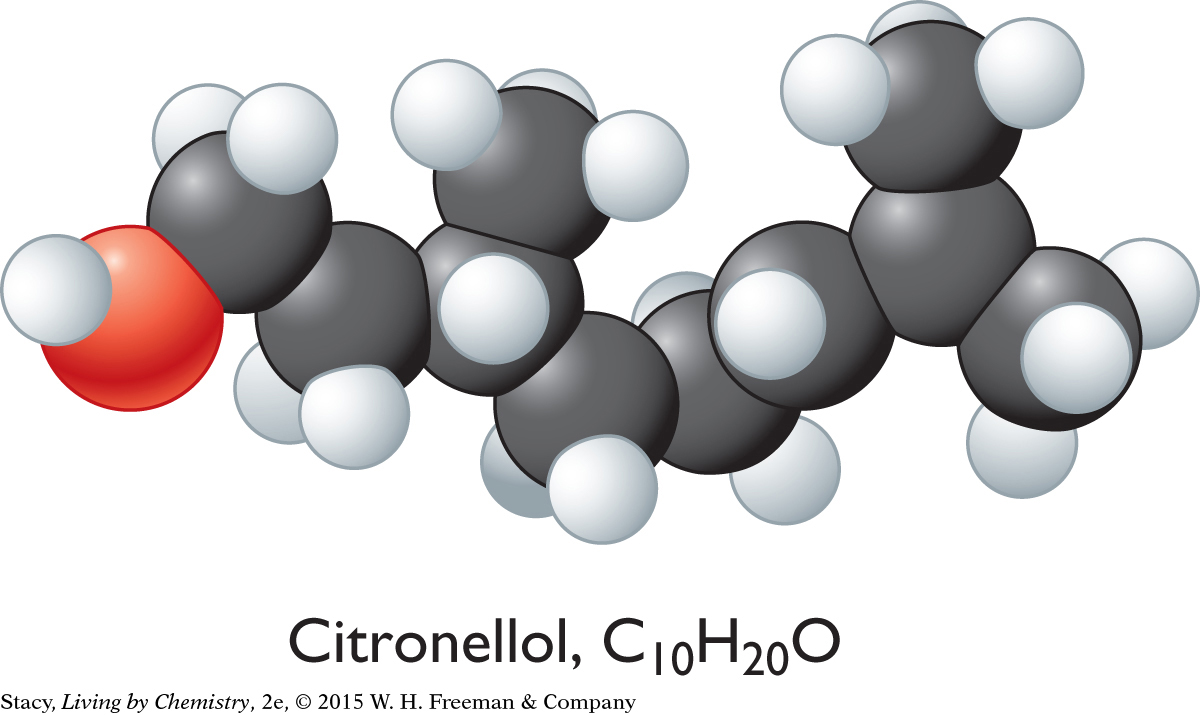
Citronellol, C10H20O
|
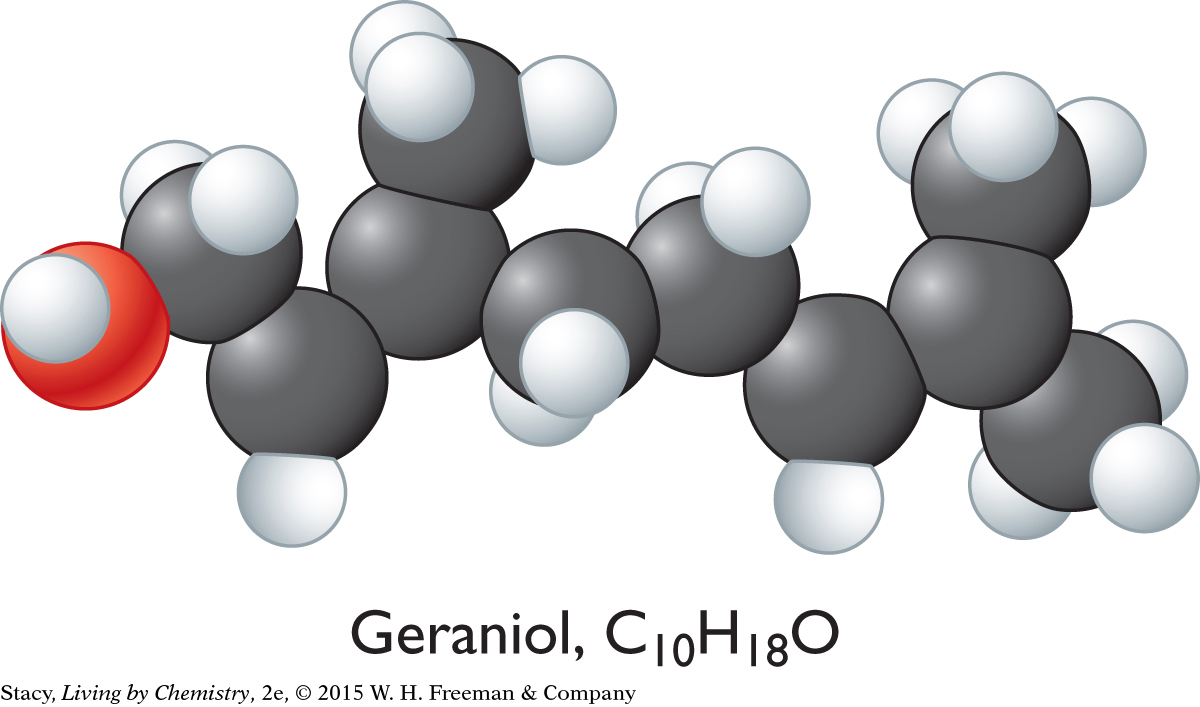
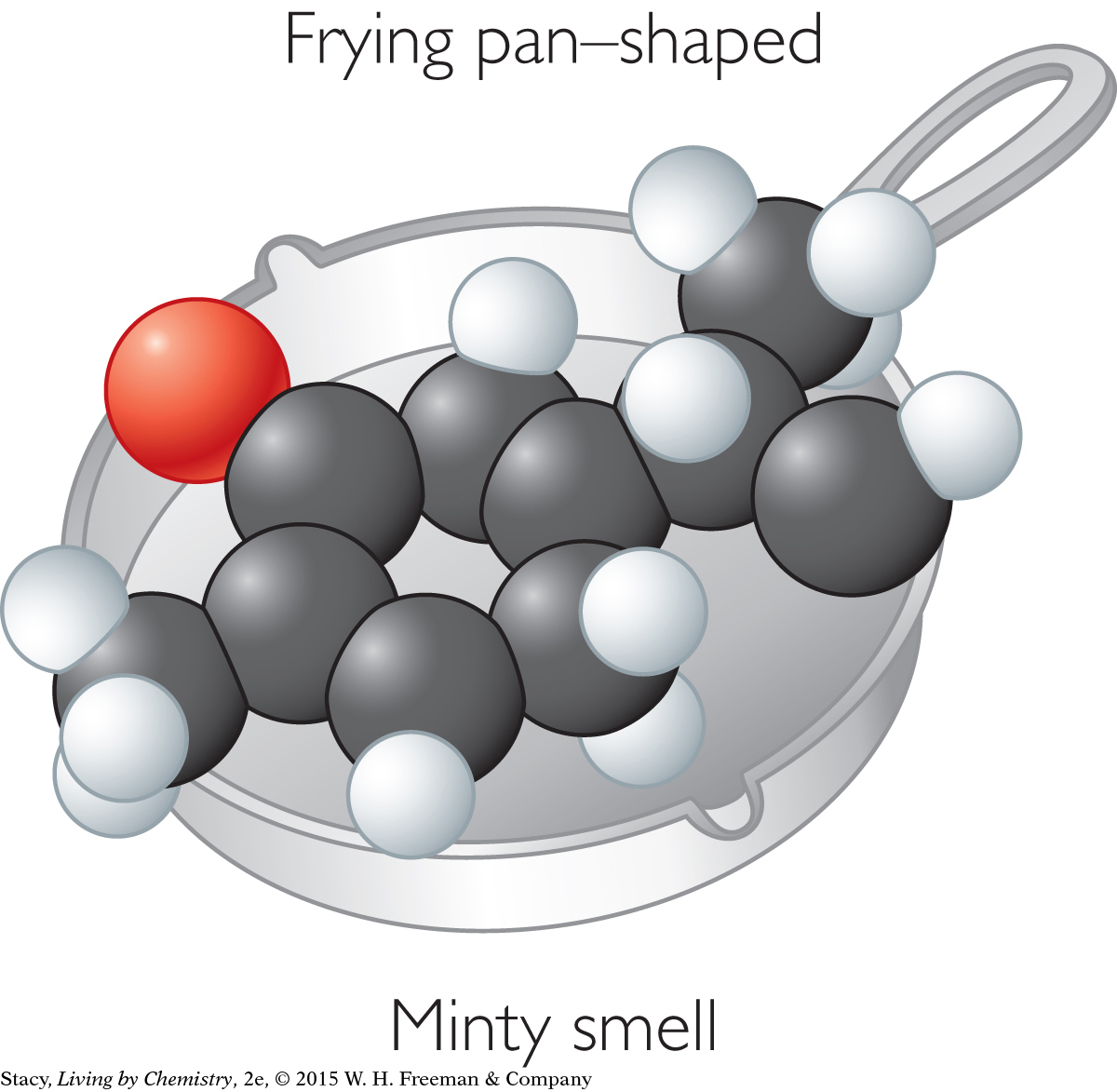
MINTY-SMELLING MOLECULES
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
Geraniol is a sweet-smelling molecule. It is one of the molecules that make roses smell like roses.

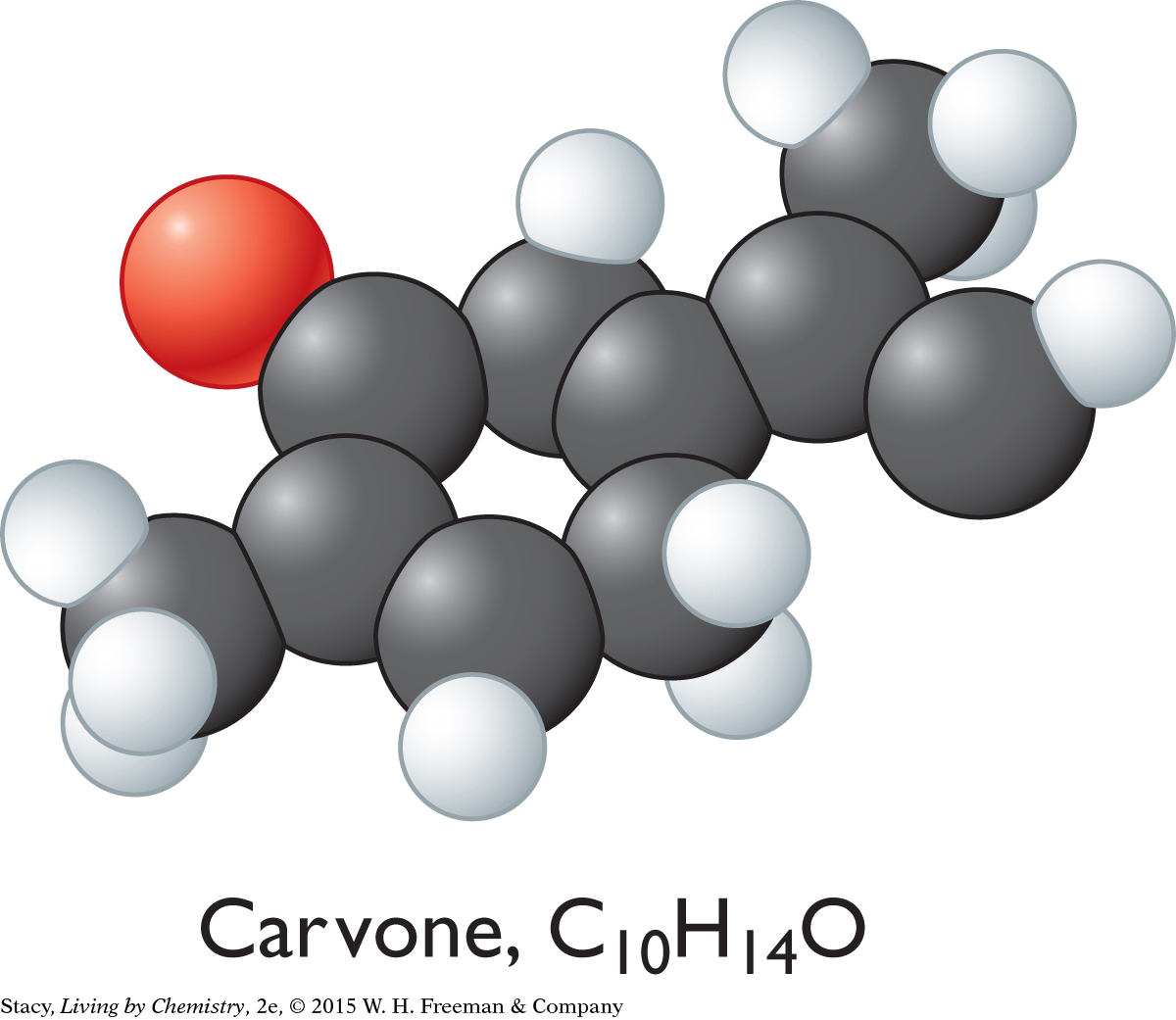
CARVONE, C10H14O
|
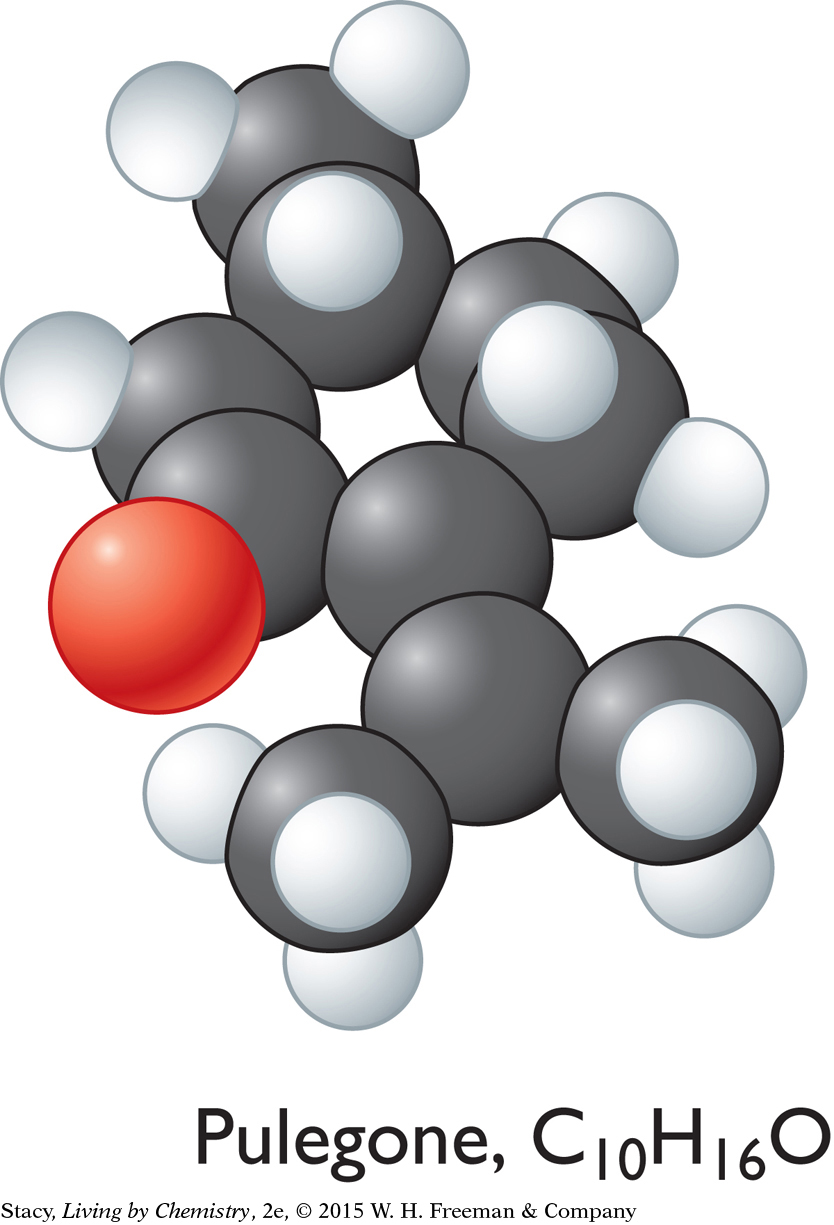
Pulegone, C10H16O
|
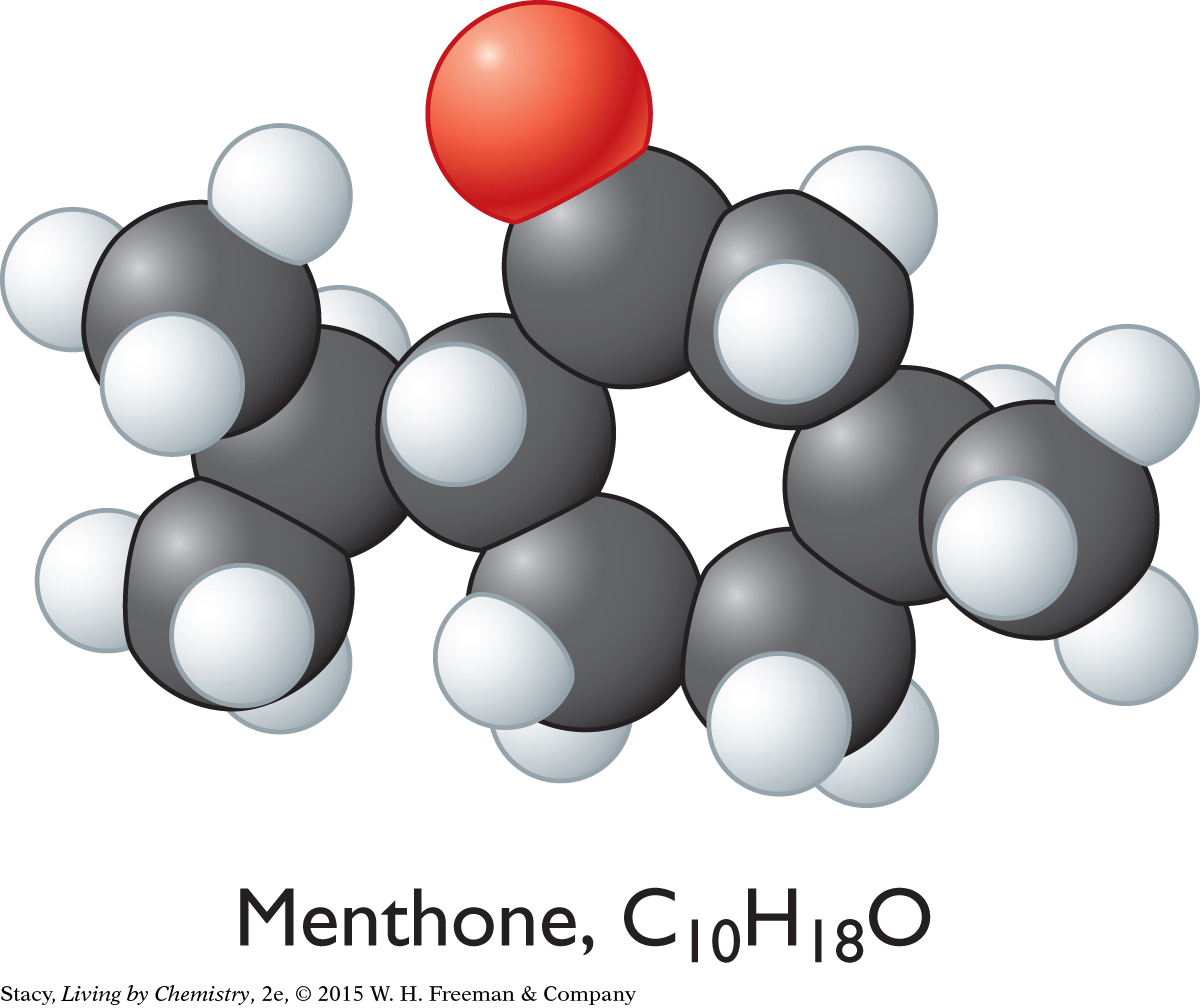
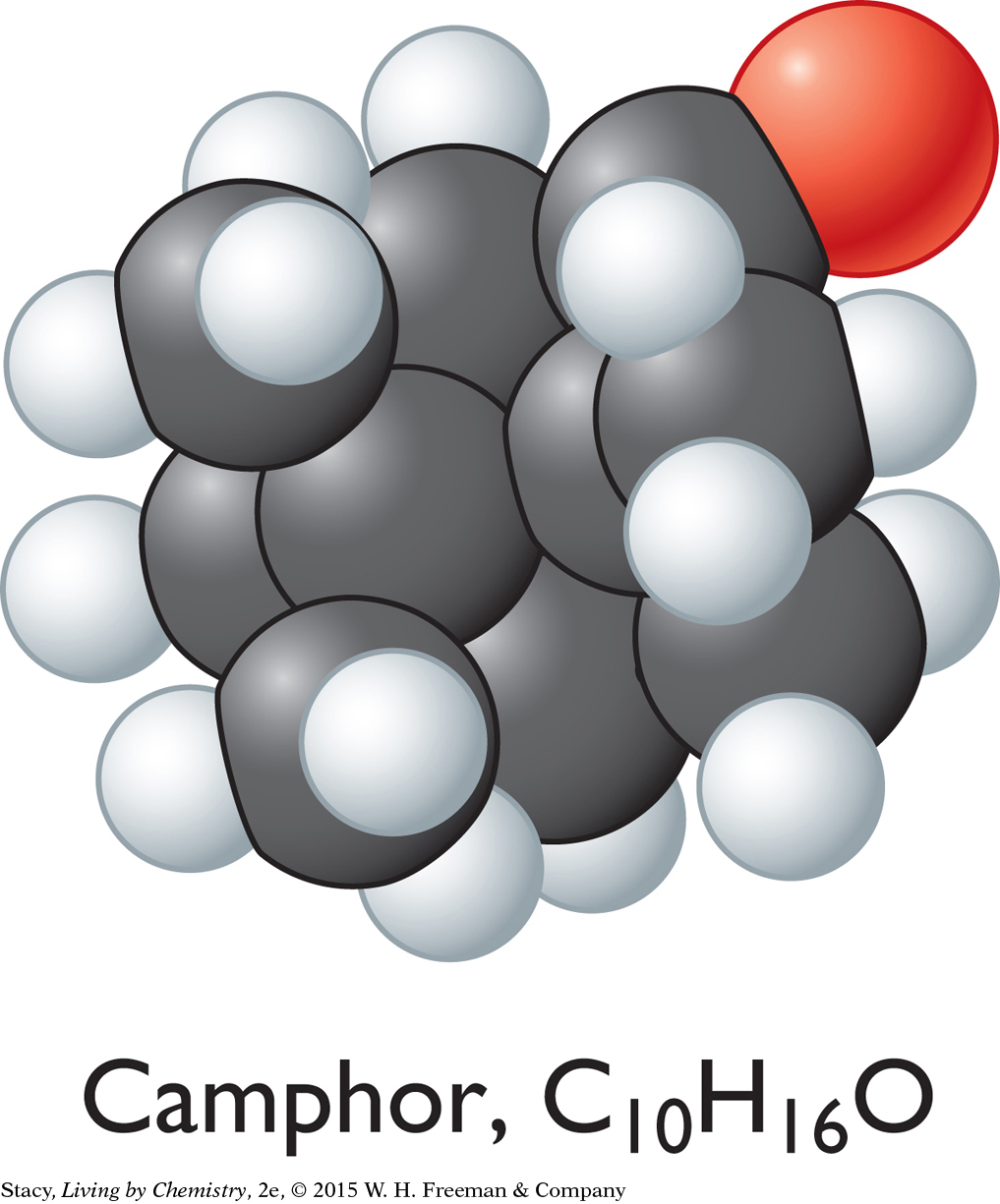
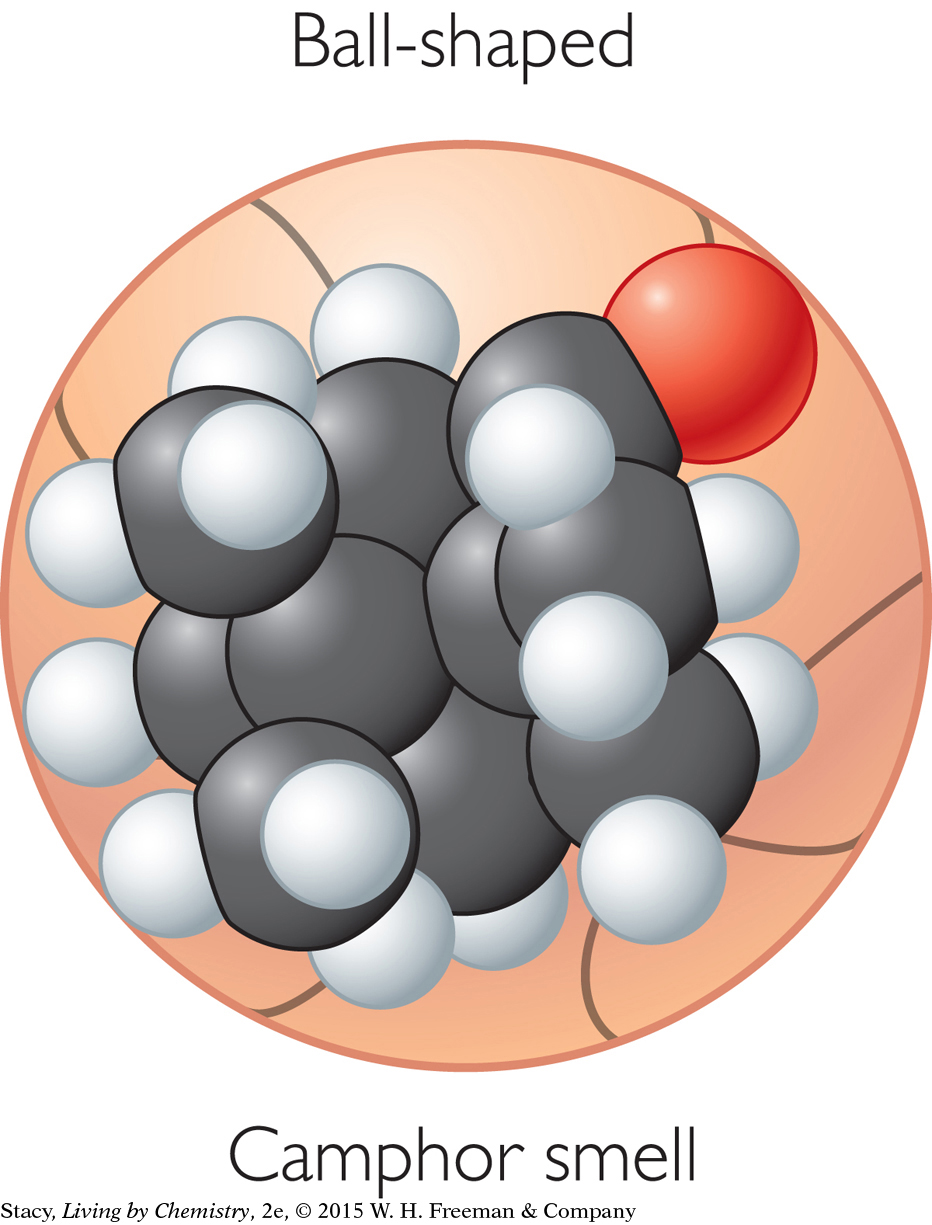
CAMPHOR-SMELLING MOLECULES
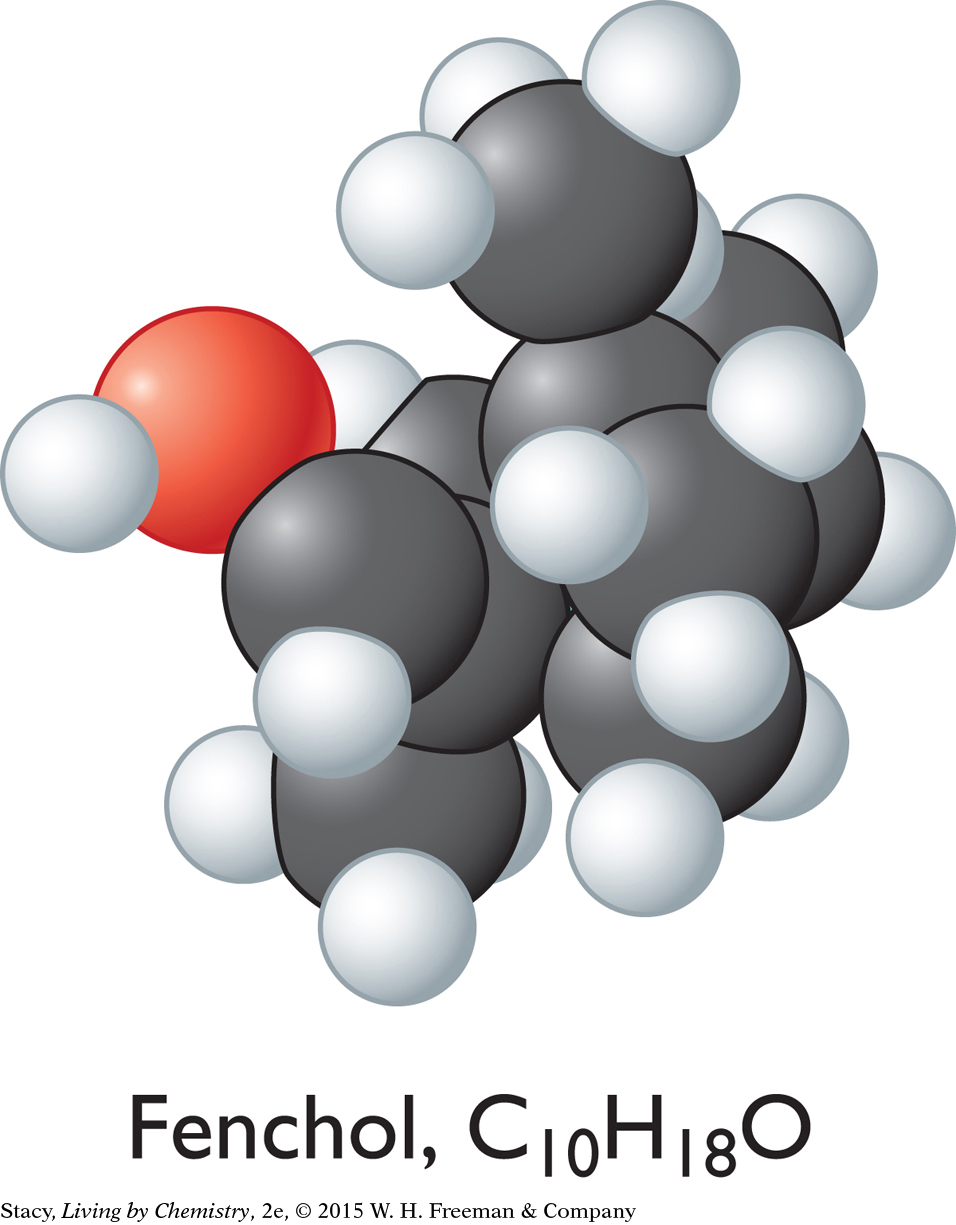
Fenchol, C10H18O
|
|
202
The sweet-smelling molecules are all long and stringy. The minty-smelling molecules all have a six-carbon ring structure. They have a shape that resembles a frying pan. The camphor-smelling molecules are a tight cluster of atoms in the shape of a ball. So far, there appear to be three smell categories that are directly related to the molecular shape.
|
|
LESSON SUMMARY
LESSON SUMMARY
How is the shape of a molecular compound related to its smell?
KEY TERM
space-filling model
A space-filling model is a three-dimensional model that shows how the atoms in a molecule are arranged in space and how they fill this space. The shape of a molecule appears to be related to its smell. For example, the sweet-smelling molecules explored here are all long and stringy in shape. The minty-smelling molecules are frying pan–shaped, and the camphor-smelling molecules are ball-shaped. More data will certainly be helpful to confirm a connection between a molecule’s shape and its smell.
203
Exercises
Reading Questions
How is a space-filling model useful?
How is a space-filling model different from a ball-and-stick model?
Reason and Apply
Draw a possible structural formula for a molecule that smells sweet and has the molecular formula C9H20O. What general shape does this molecule have?
If someone told you a molecule had a six-carbon ring, what smell would you predict for that compound?
Draw a possible structural formula for a molecule that smells minty and has the molecular formula C10H16O. What general shape does this molecule have?
If someone told you a molecule is ball-shaped, what else would you want to know to predict the smell of the molecule?
Which do you think has more influence on smell: the functional group that is present or the shape of a molecule? Explain why you think so.