LESSON 41: How Does The Nose Know?: Receptor Site Theory
208
THINK ABOUT IT

Imagine you are sipping a cool glass of lemonade on a hot summer day. The lemonade contains some fresh mint leaves for extra flavor. You detect both the lemon smell and the minty smell. The molecules associated with these two smells are quite different. What is happening inside your nose that allows you to detect both smells at the same time and to tell them apart? How does the nose know these molecules are different?
How does the nose detect and identify different smells?
To answer this question, you will explore
Receptor Site Theory
Phase Change and Molecular Stability
Receptor Site Theory
EXPLORING THE TOPIC
Receptor Site Theory
HEALTH CONNECTION
HEALTH
CONNECTION
Many medications work by using a lock and key mechanism. The molecules found in a medication may be designed to stimulate a receptor site, resulting in the desired physical response. Or a medication may have been designed to block receptor sites, preventing a certain unwanted physical response.
The chemistry of smell is a fairly new science, and much remains to be discovered about how the sense of smell works. A number of theories exist to explain what happens in the nose when a smell is detected. While scientists are still uncertain about all of the details about how smell works, one theory seems to fit the evidence better than the others. This theory is called the receptor site theory. It is a widely accepted theory of how the interior of the nose detects different smells.
The receptor site theory uses the “lock and key” model. In this model, molecules are like “keys” that fit only into certain “locks.” In other words, molecules have specific shapes that will fit only certain receptor sites, and the lining of the nose is covered with receptor sites.
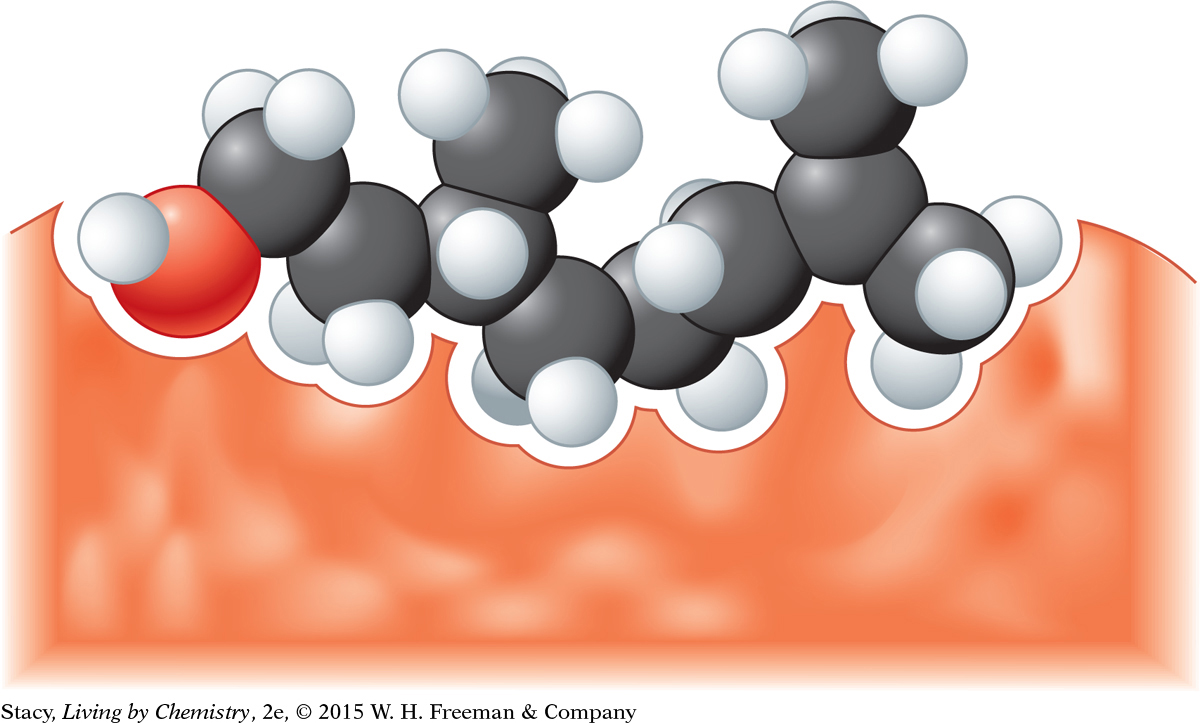
The lemony-smelling and minty-smelling molecules in the compounds of the lemonade might fit into different receptor sites as shown in the illustration below.
209
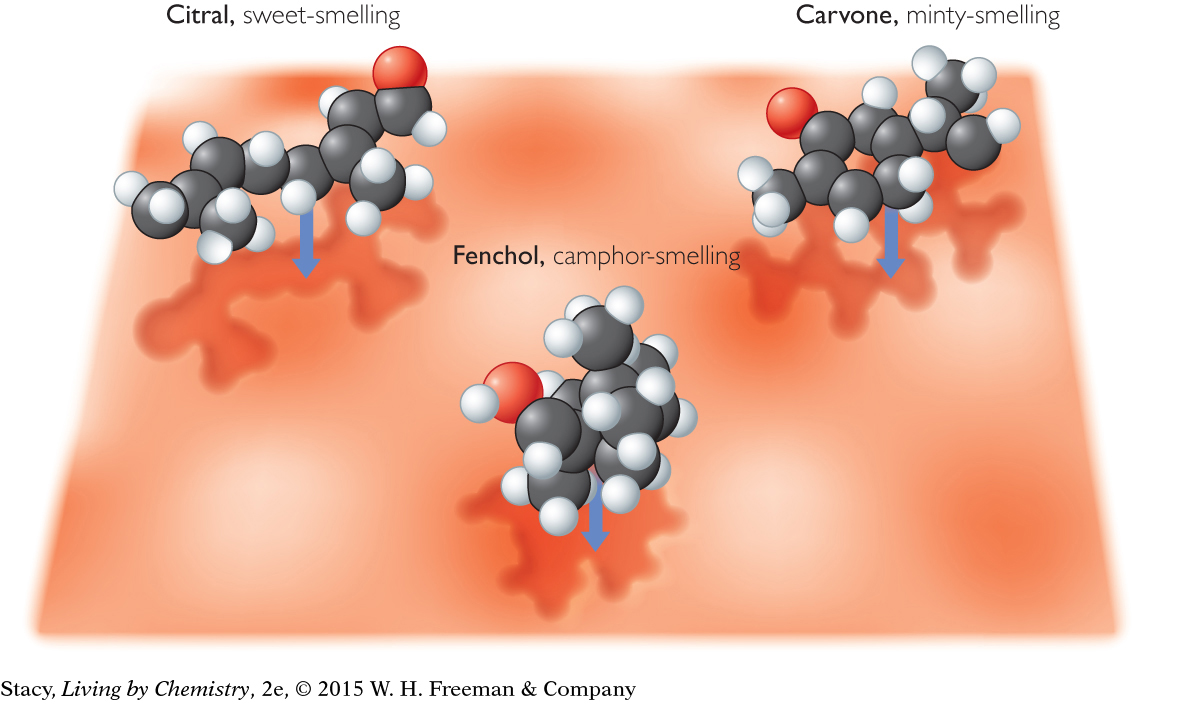
In this model, each type of molecule fits only into its own receptor sites, not into the others. According to receptor site theory, this is how the nose distinguishes between two molecules.
Scientists are unsure how many receptor sites are in the nose or how many different shapes the sites accommodate. However, they do know that receptor sites are made of large, very intricate protein molecules. These protein molecules consist of hundreds of atoms bonded together. They are much more elaborate and complex than the simple drawings of receptor sites shown in the illustration.
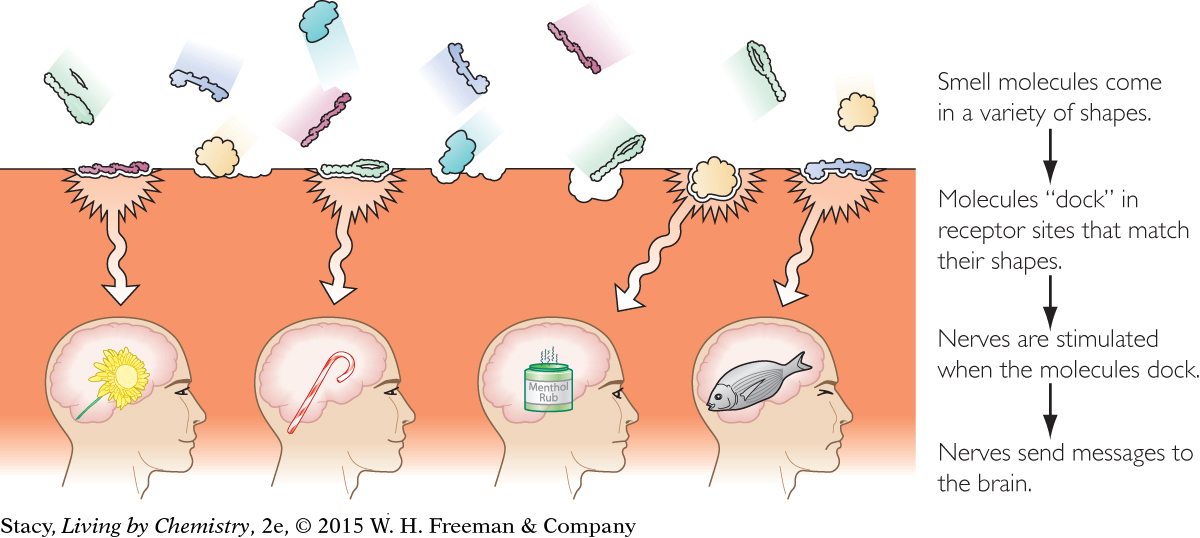
Scientists think that once a smell molecule has “docked” in a receptor site, nerves are stimulated and send a message to the brain. If minty receptor sites are stimulated, then the brain registers a minty smell.
It is possible that smell is much more complex than just described. A specific smell might be the result of a combination of different molecules stimulating a combination of receptor sites.
Phase Change and Molecular Stability
Phase Change and Molecular Stability
210
CONSUMER CONNECTION
CONSUMER
CONNECTION
A chocolate bar looks solid. Still, we can smell chocolate, so we know that some of the molecules that make up chocolate must be changing phase to become a gas and entering the nose. If you melt a chocolate bar, the scent will be stronger because more molecules are escaping in the gas phase.

SMELLS ARE GASES
When you bring an onion home from the supermarket, it doesn’t give off much of a smell. But when you slice or chop an onion, the odor can become quite overpowering. What’s happening? When you cut up the onion, your knife is cutting through some of the cell walls of the onion and releasing a lot of liquid.
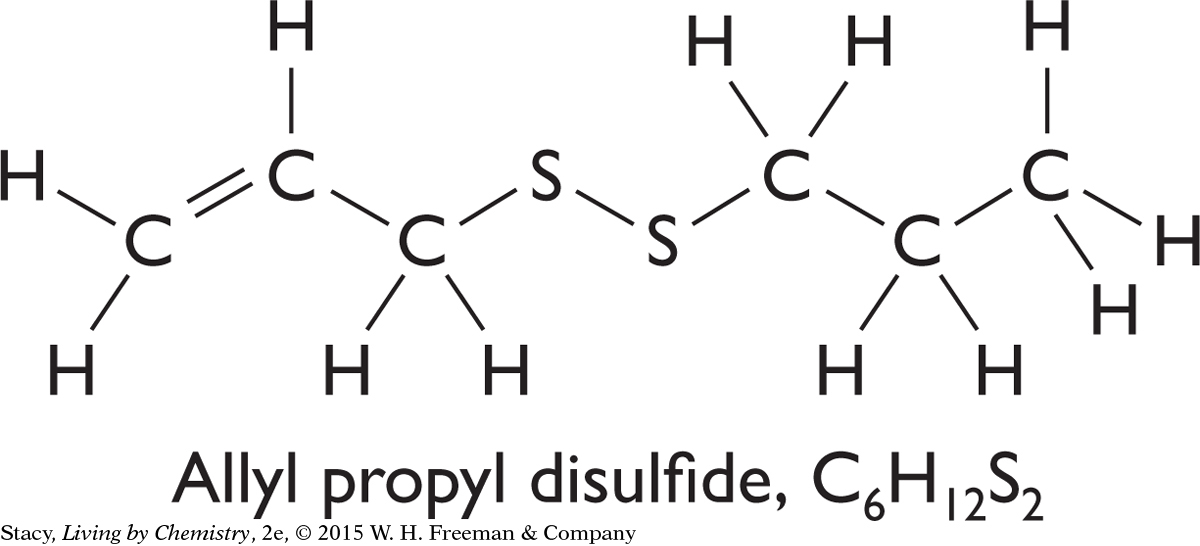
One of the molecules that is in this liquid and is responsible for the distinctive smell of onions is allyl propyl disulfide. Its structural formula is shown here. Its molecular formula is C6H12S2. This is the molecule your nose detects when you smell onions.

© Chris Elwell/iStockphoto
|
|
But something else has to happen for you to smell the onion. Molecules of allyl propyl disulfide have to get from the onion into your nose. The only way that can happen is if some of the molecules travel through the air to your nose. To do this, the compound changes phase to become a gas or vapor that floats through the air and enters your nostrils. There is no other possible explanation for your ability to smell an onion several feet away.
Important to Know
A substance does not have to boil in order for some of its molecules to become a gas. At the surface of molecular liquids and solids, some molecules escape and become a gas.
You cannot smell something unless some portion of it is in the form of a gas. This means that everything you are able to smell is a substance that has become a gas.
MOLECULES ARE STABLE UNITS
Because your nose is detecting the shape and functional group of a molecule, this means that the molecule must enter your nose in one piece. When molecules change phase, they do not break apart into pieces or into individual atoms. Molecules go from the solid phase, to the liquid phase, to the gas phase without breaking apart into individual atoms. This is because molecules are stable units, with strong covalent bonds between the atoms. For example, water is still water whether it is in the form of water vapor, liquid water, or ice.
Big Idea
Big Idea
Molecules are stable units. They do not break apart when they change phase.
LESSON SUMMARY
LESSON SUMMARY
How does the nose detect and identify different smells?
KEY TERM
receptor site theory
The receptor site theory explains how the nose detects different smells. This theory suggests that smell molecules travel to receptor sites in the lining of the nose. Once a smell molecule docks in a receptor site, it stimulates a nervous-system response, sending a signal to the brain. Everything you smell is in the form of molecules in the gas phase.
211
Exercises
Reading Questions
Explain the receptor site theory and how it applies to smell detection.
What does phase change have to do with smell?
Reason and Apply
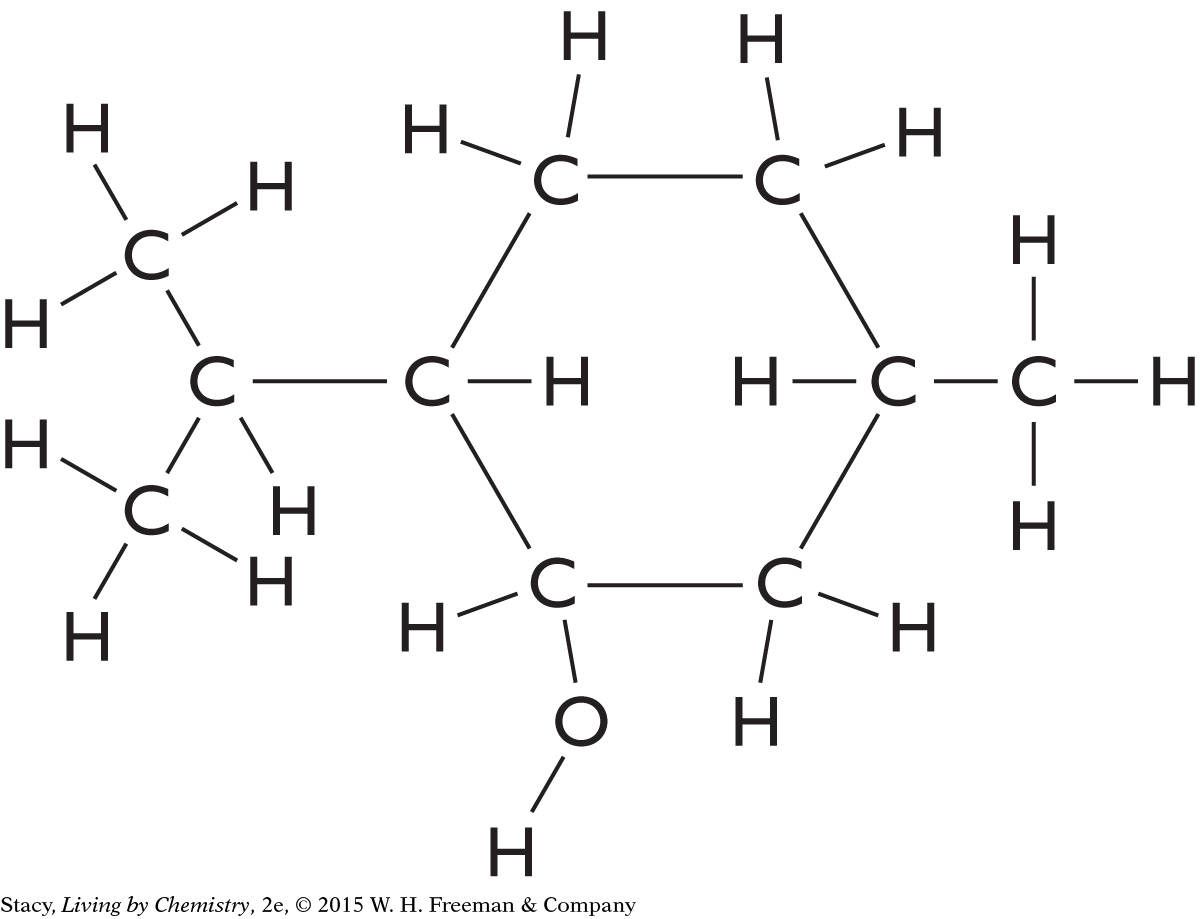
Here is a structural formula for an active ingredient in muscle ointment. How does this compound smell? How does your nose detect the smell?
A chemist creates a new molecule that has a completely different three-dimensional shape from other molecules humans have ever encountered. Would you be able smell it? Select the best answer.
Probably not, because synthetic compounds do not have a smell.
Definitely, because all esters smell sweet.
Probably not, because our noses would not have developed receptor sites to detect it.
Definitely, because parts of the molecule can break off to fit into receptor sites in our noses.
Some smells are similar, such as popcorn and freshly baked bread. Explain how you might account for similar smells using the receptor site theory.
How might the receptor site theory explain why a dog has a better sense of smell than a person?
Why do you think you might “get used to” a smell and hardly detect it? Use the receptor site model in your explanation.
Are some smells “faster” than others? Explain.