LESSON 45: I Can Relate: Polar Molecules and Smell
230
THINK ABOUT IT
Polar molecules have a potent smell. The smell of ammonia, NH3, in some window cleaners is quite strong. In contrast, the methane gas, CH4 does not have a smell even though its C–H bonds are all polar. So, why can’t you smell CH4 gas?
What does polarity have to do with smell?
To answer this question, you will examine
Polarity of Molecules
Nonpolar Molecules
Polarity and Smell
Polarity of Molecules
EXPLORING THE TOPIC
Polarity of Molecules
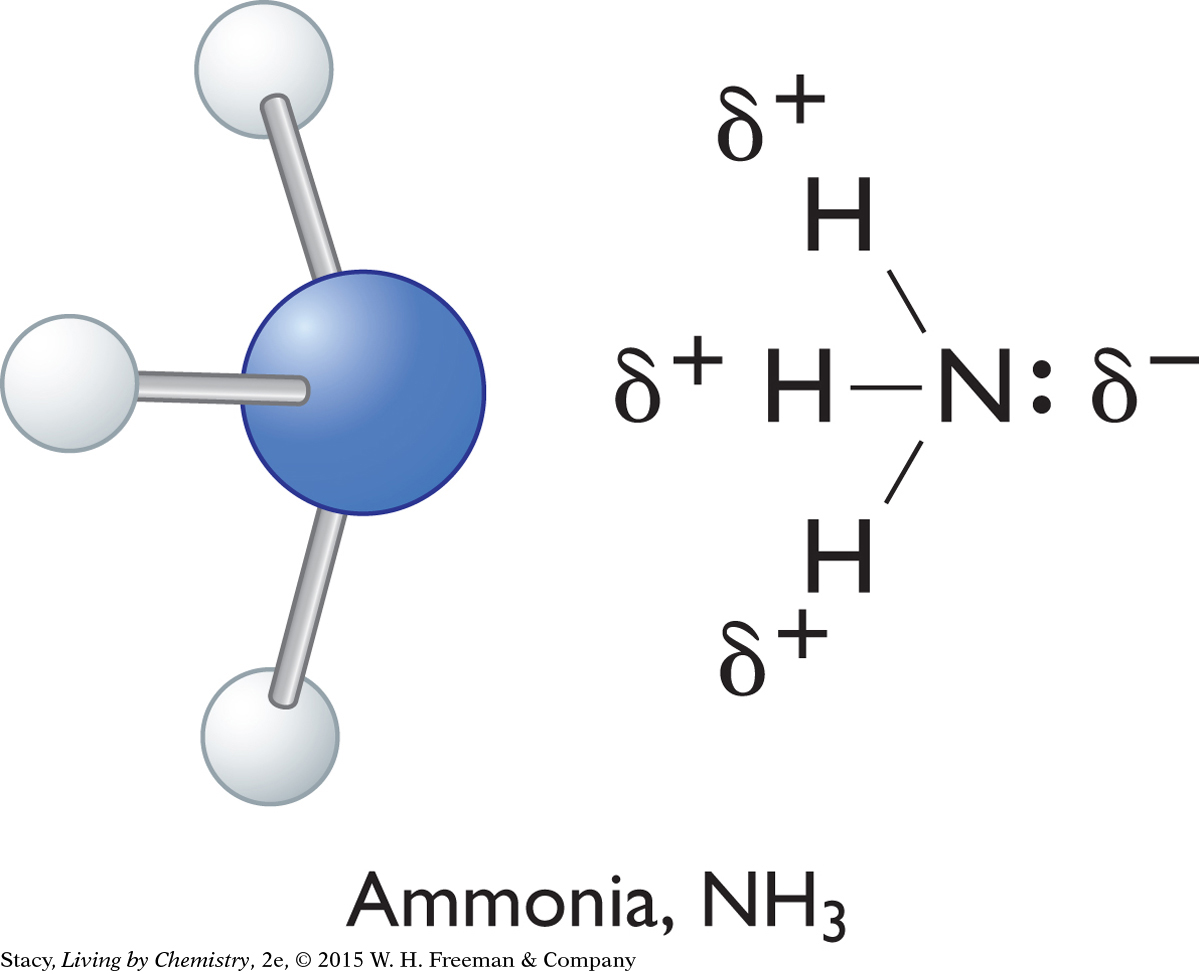
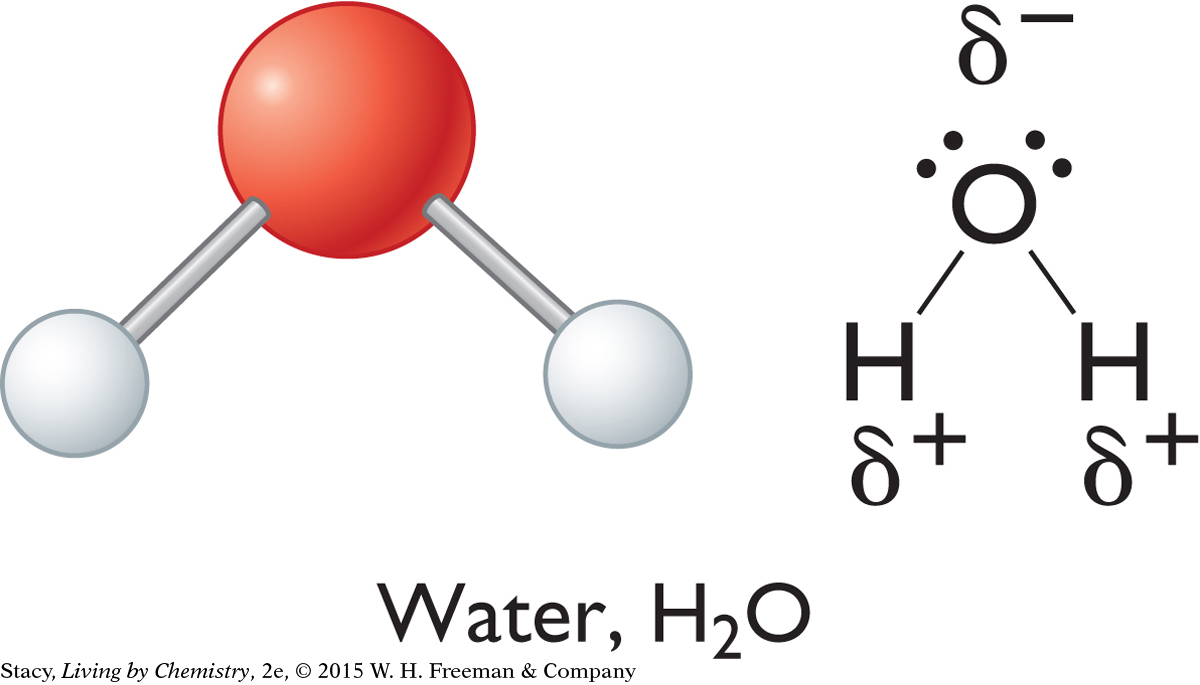
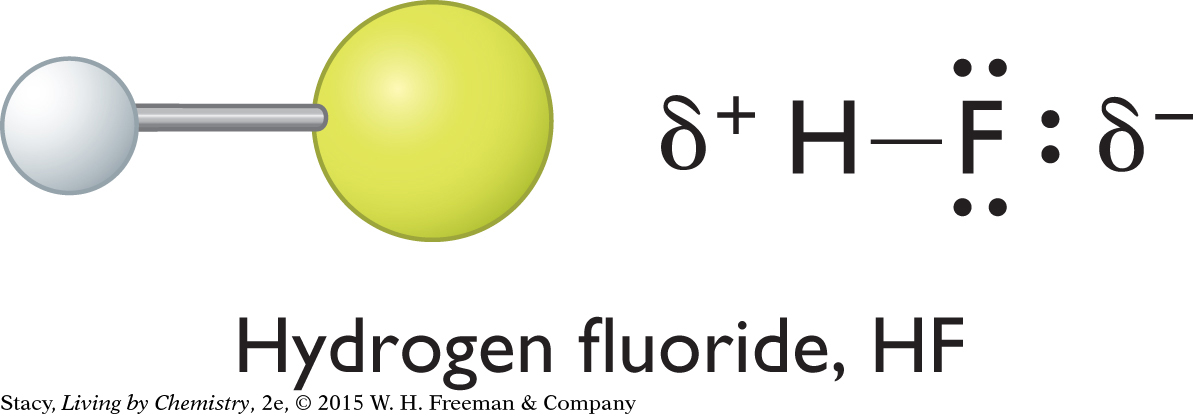
If the bonds between atoms of a molecule are polar, what about the molecule as a whole unit? One way to tell whether a molecule is polar is to examine its overall shape. Notice that each of the three molecules shown here has an irregular shape, or some sort of asymmetry. Asymmetric molecules are polar.
|
|
|
Ammonia, water, and hydrogen fluoride are all polar. In these molecules, there is a partial negative charge on the nitrogen, oxygen, and fluorine atoms because these atoms are more electronegative than hydrogen atoms. The hydrogen atoms have partial positive charges. To figure out which end of a molecule has a partial negative charge and which end has a partial positive charge, check the electronegativities of the atoms and the overall shape of the molecule.
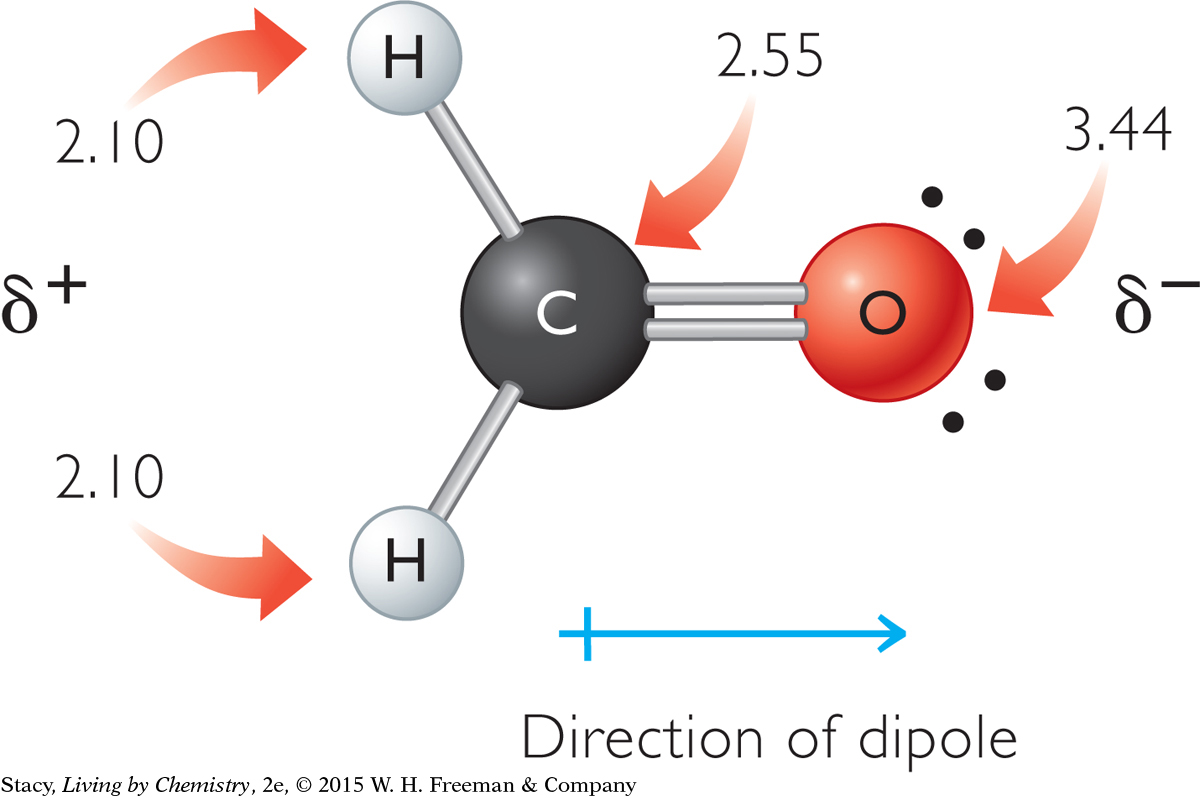
Consider formaldehyde, CH2O, shown here. The numbers next to the atoms are their electronegativities.
231
The oxygen end of the formaldehyde molecule has a partial negative charge because the oxygen atom is the most electronegative atom in the formaldehyde molecule. Similarly, the hydrogen atoms have partial positive charges because they are the least electronegative atoms in the molecule.
Example 1
Phosphine, PH3
Is phosphine, PH3, polar? What is the direction of the dipole?
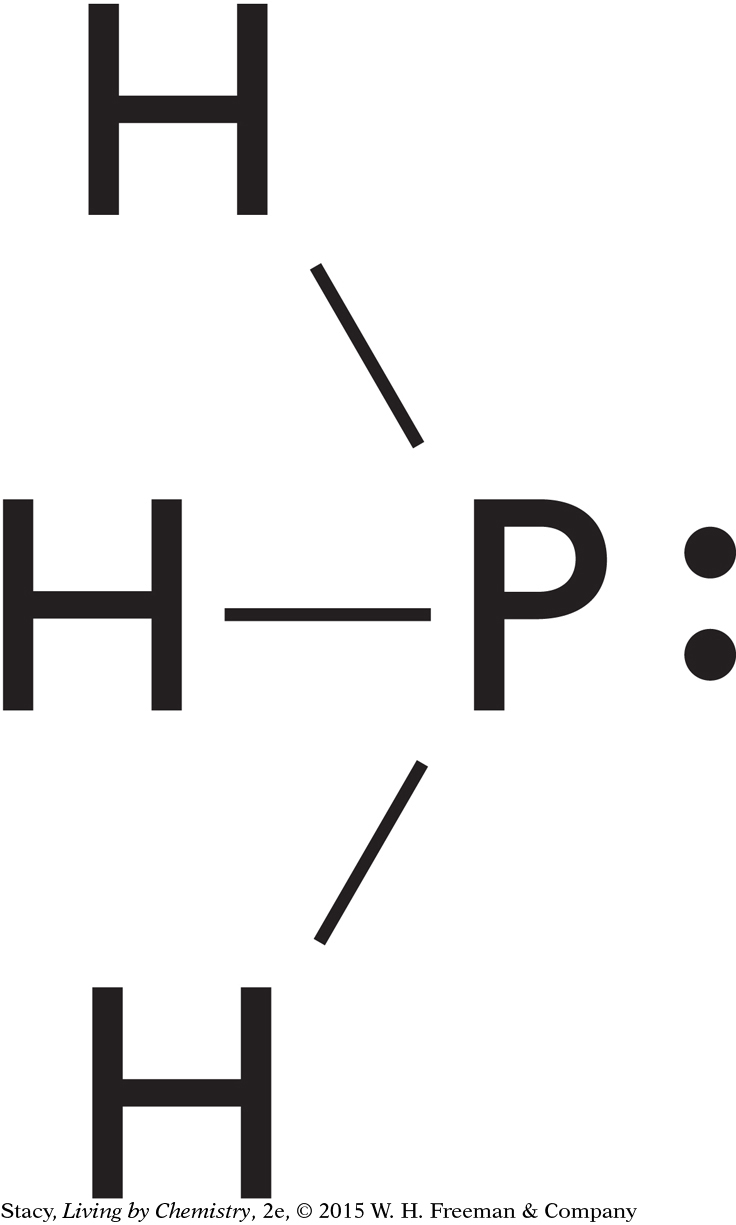
Solution
A Lewis dot structure of PH3 shows four pairs of electrons, three bonding pairs and one lone pair. These four pairs of electrons are arranged around the phosphorus, P, atom in a tetrahedral shape.
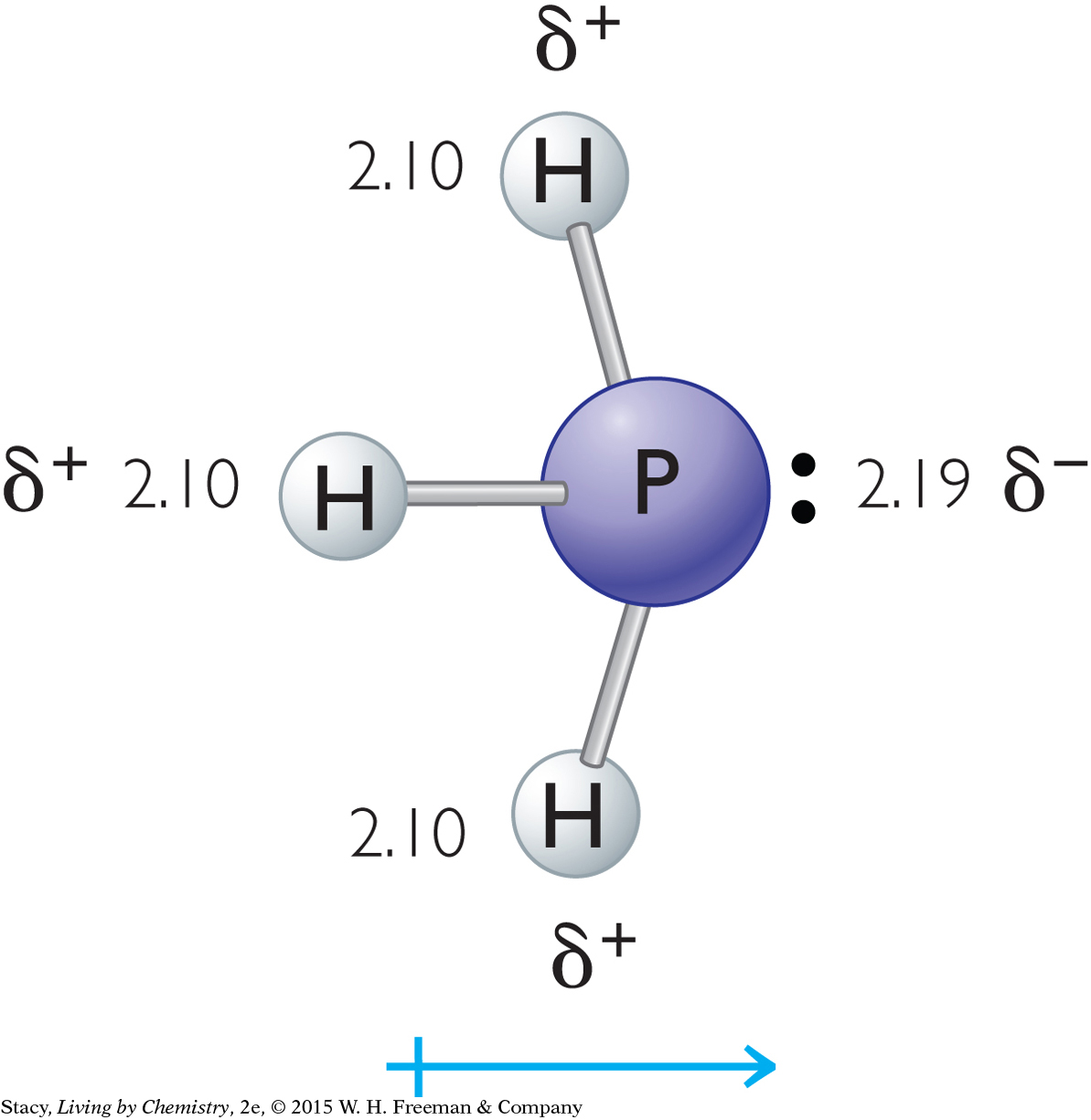
Because the molecule is asymmetrical, it is polar. You can find the electronegativity values for phosphorus and hydrogen in the electronegativity scale in Lesson 44: Thinking (Electro)Negatively. The electronegativity value for P is 2.19 and for H is 2.10. Because the electronegativity of the phosphorus atom is greater, the P atom attracts the electrons more strongly and has a partial negative charge. The H atoms have partial positive charges. The overall dipole is shown.
Nonpolar Molecules
Nonpolar Molecules
Diatomic molecules with two identical atoms are nonpolar. The electrons between the two identical atoms are shared equally. However, these are not the only kind of nonpolar molecules. Some molecules are symmetrical. The symmetry in these molecules can make them nonpolar even though they have polar bonds within them. The polarities of the individual polar bonds balance each other out due to the shape of the molecule.
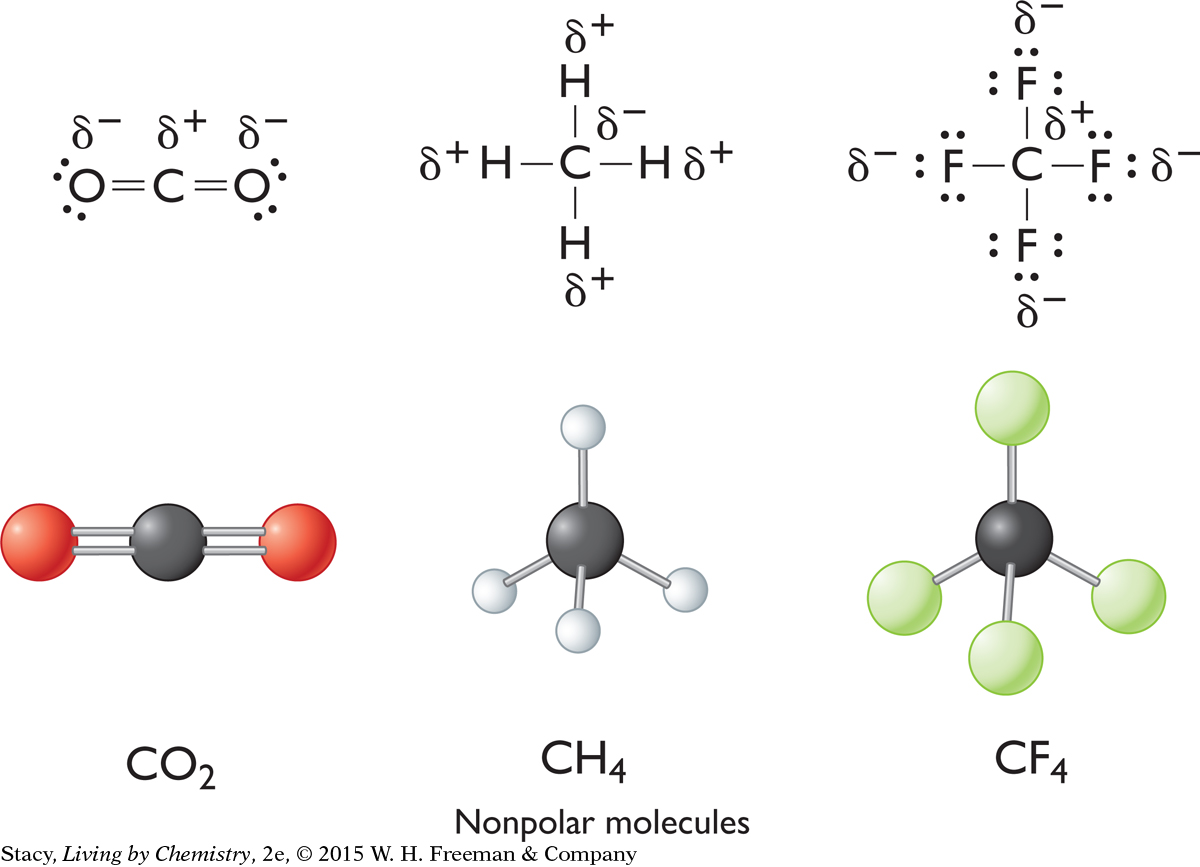
Polarity and Smell
Polarity and Smell
232
Small molecules that are polar have a smell. Small molecules that are nonpolar do not have a smell. What does polarity have to do with the smell of small molecules?
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
Chlorotrifluoromethane, also known as Freon 13, was developed early in the 20th century and used widely as a refrigerant. However, use of Freon 13 and other chlorofluorocarbons (CFCs) was significantly reduced in the late 1980s due to their effects on the ozone layer of the atmosphere.


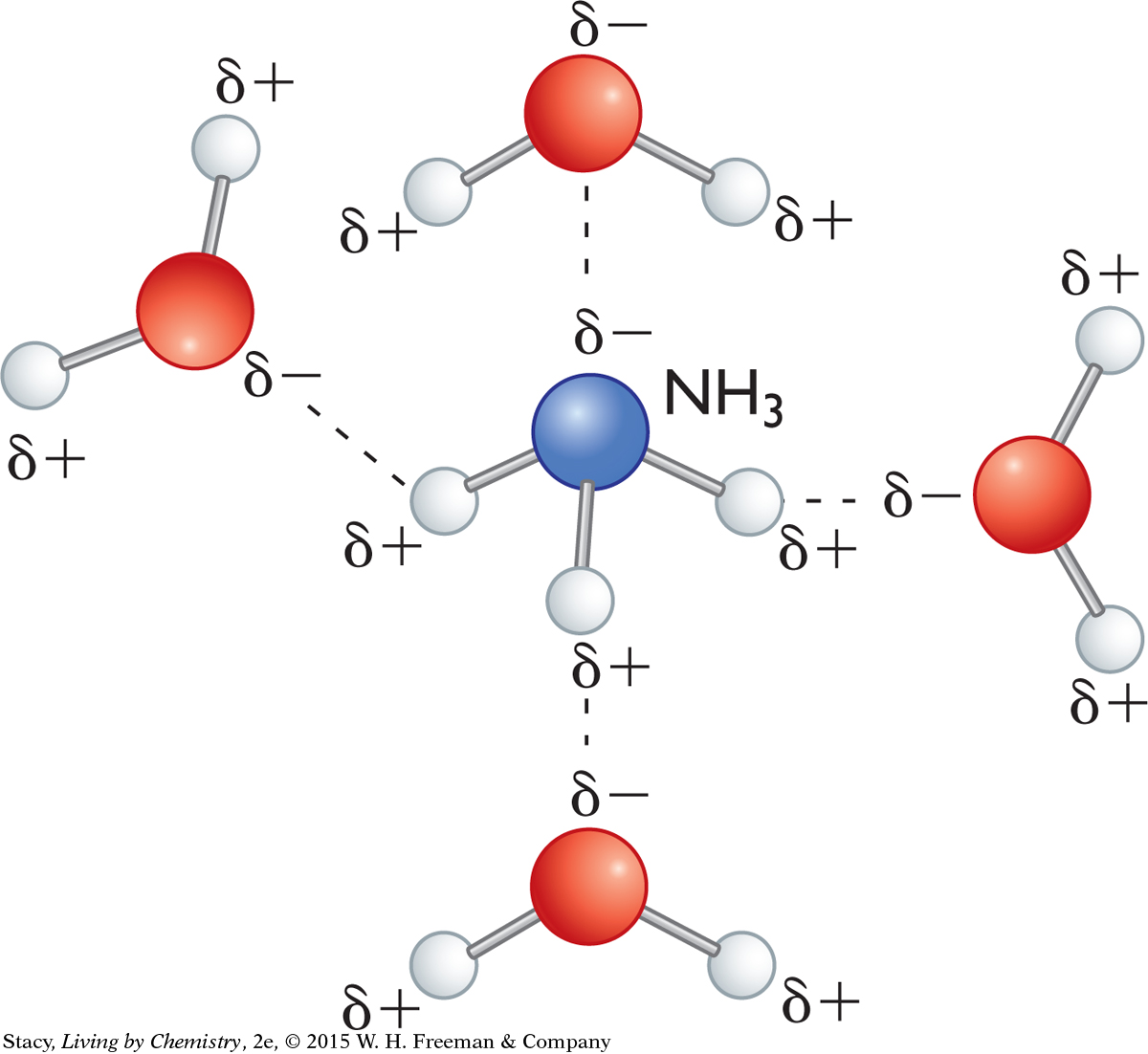
Polar molecules have properties that distinguish them from nonpolar molecules. Polar molecules dissolve in water and are attracted to other polar molecules. One well-accepted theory is that polar molecules dissolve in the mucous membrane of the nose and then attach to receptor sites. The mucus is composed largely of water.
RECEPTOR SITES IN THE NOSE
Receptor sites in the nose contain large polar protein molecules. Scientists believe that small polar molecules are attracted to the polar receptor sites after they enter the nose. You could think of polarity as working somewhat like a magnet, with small polar molecules “sticking” to the polar part of a receptor site. Nonpolar molecules are not attracted to the same extent as polar molecules, so they may not be detected by the nose.
Example 2
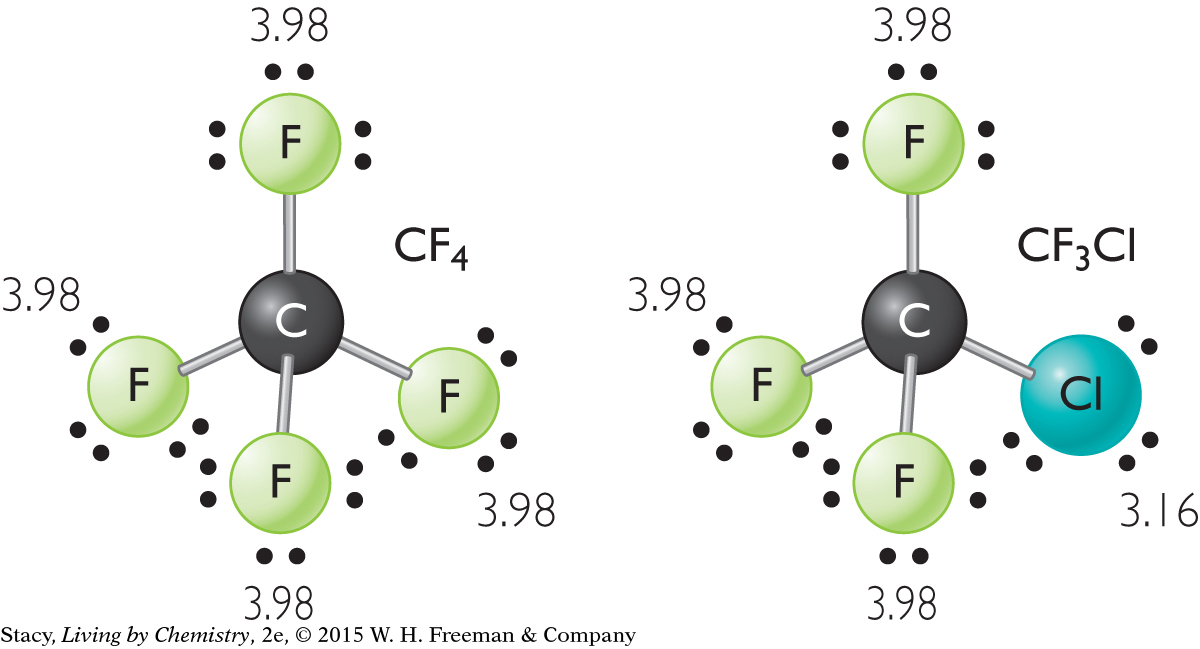
Chlorotrifluoromethane, CClF3, and Tetrafluoromethane, CF4
Compare chlorotrifluoromethane with tetrafluoromethane. Explain why one of these molecules has a smell and the other does not.
Solution
Both molecules have a tetrahedral shape. In tetrafluoromethane, each C–F bond is polar. However, the four C–F bonds are distributed symmetrically so that the molecule as a whole is nonpolar. Therefore, it does not have a smell.
Chlorotrifluoromethane is not as symmetrical. This is because one of the F atoms is replaced by a Cl atom. The molecule is polar because F and Cl have different electronegativities. Therefore, it has a smell.
LESSON SUMMARY
LESSON SUMMARY
233
What does polarity have to do with smell?
NATURE CONNECTION
NATURE
CONNECTION
Many animals, such as elephants, seem to be able to smell water at great distances.

Small asymmetrical molecules with polar bonds are polar. Molecules that are symmetrical in every way are nonpolar. Theories suggest that the polarity of a molecule may help it to “stick” in a receptor site in the nose. Further, polar substances are more soluable in water. This may allow polar substances to dissolve in the mucous membrane of the nose, whereas nonpolar substances are simply exhaled undissolved and undetected.
Exercises
Reading Questions
How can you determine if a molecule is polar?
Describe one theory of why small nonpolar molecules do not have a smell.
Reason and Apply
For each of the molecules listed, draw a Lewis dot structure and indicate the shape of each molecule. Decide whether these substances smell. Explain your reasoning for each.
H2Se
H2
Ar
HOF
CHClF2
CH2O
For each of the polar molecules in Exercise 3, draw the dipole.
Water is an exception to our rule about small molecules. It is a polar molecule, yet humans don’t smell it. What do you think is going on?
Do you think it might be useful if you could smell the air? Explain your thinking.
Methane, CH4, gas leaks can be very dangerous and can be explosive. Why do you think dimethyl sulfide, C2H6S, is added to natural gas that is used in homes and buildings?