LESSON 46: Sniffing It Out: Phase, Size, Polarity, and Smell
234
THINK ABOUT IT
You can smell chicken frying or someone’s perfume from across a room, but you can’t smell a piece of paper or a binder on your desk. And cookies right out of the oven are much easier to smell than cold ones. How can you use what you have learned about molecules to understand these observations?
What generalizations can you make about smell and molecules?
To answer this question, you will explore
Bonding and Smell
Molecular Size and Smell
Bonding and Smell
EXPLORING THE TOPIC
Bonding and Smell
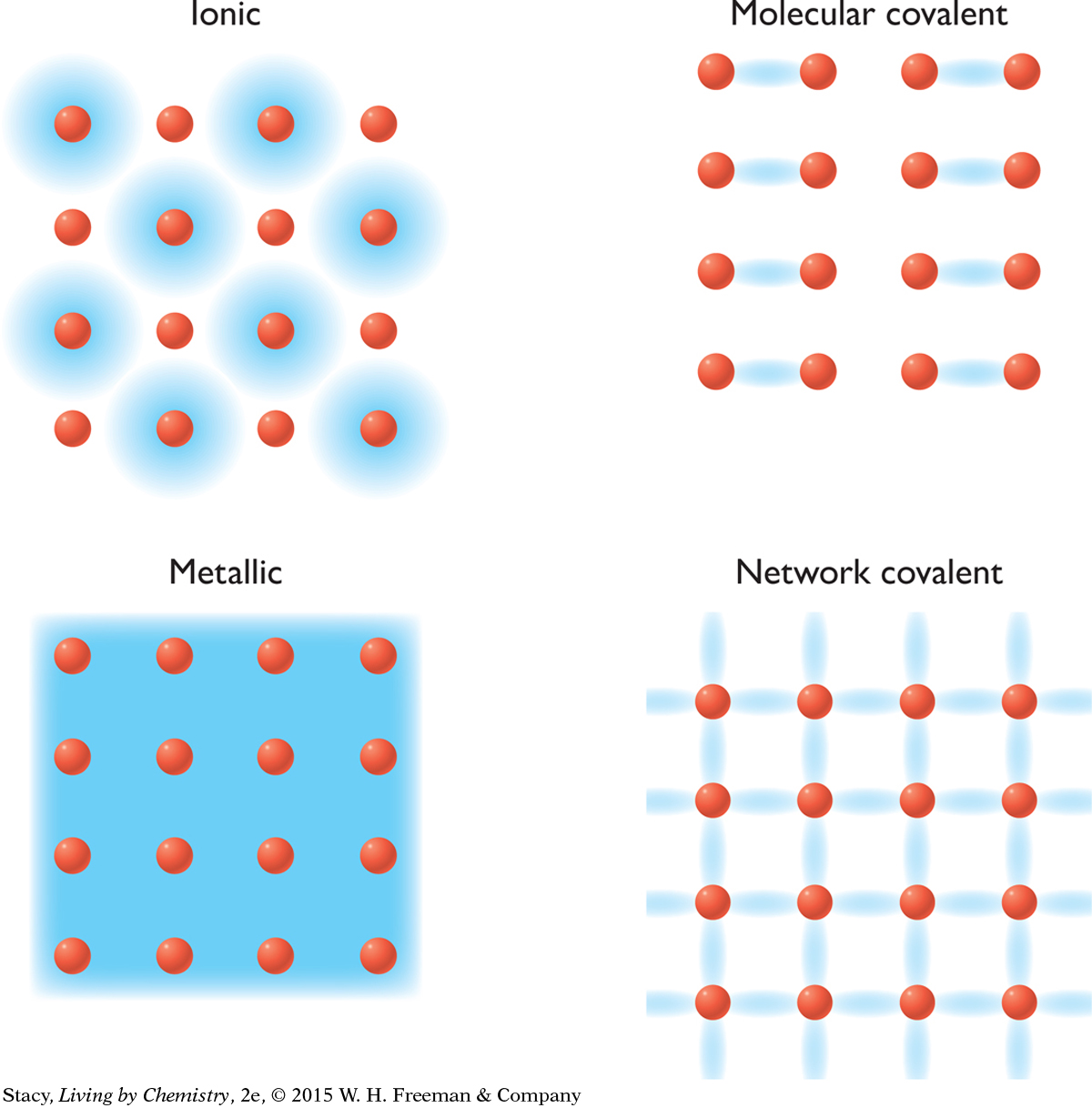
In Unit 1: Alchemy, you sorted matter into four classes based on whether the substance dissolved in water and whether the substance conducted electricity. This sorting led to four models of bonding: ionic, molecular covalent, metallic, and network covalent. How does the property of smell relate to bonding?
235
Recall that all the substances you smelled in this unit were molecules with nonmetal atoms. This evidence suggests that substances that have a smell fit into the molecular covalent category.
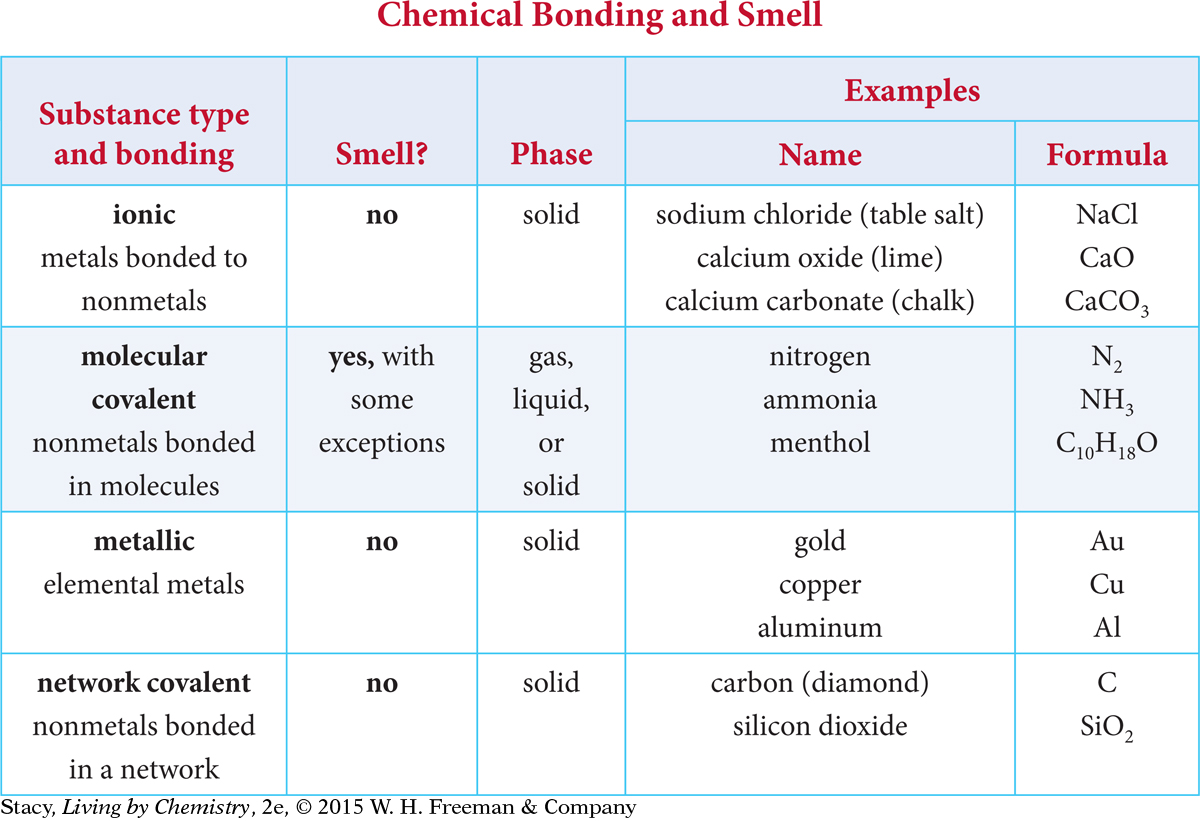
Molecular covalent substances exist as gases, liquids, or solids at room temperature. Substances in the other three bonding categories are strictly solids (with a few exceptions, such as liquid mercury). It is reasonable to propose that substances that are ionic, metallic, or network covalent do not have a smell because they do not form gases easily at room temperature. This means that they cannot get into the receptor sites in our noses.
Big Idea
Big Idea
Only molecular covalent substances form gases at room temperature.
Example 1
Bonding and Smell
Predict whether you would be able to smell the following substances. Explain your reasoning.
propanol, C3H8O
iron, Fe
copper sulfate, CuSO4
Solution
Propanol consists of nonmetal atoms, so it is a molecular covalent substance. It probably has a smell.
Iron is a metallic solid. It does not have a smell.
Copper sulfate consists of both metal and nonmetal atoms, so it is an ionic solid. It does not have a smell.
Molecular Size and Smell
Molecular Size and Smell
236
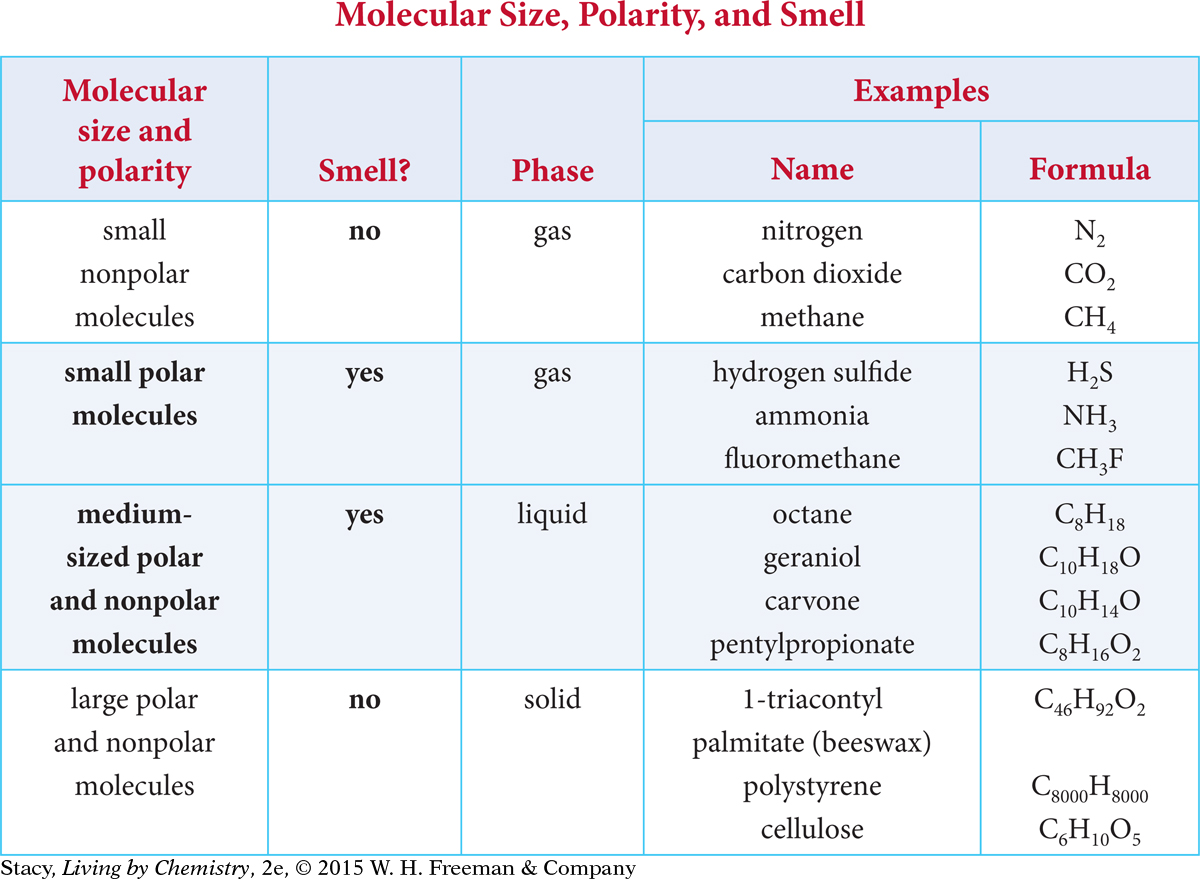
In the previous lesson, you learned that small molecules have a smell only if they are polar. What about medium-sized and large molecules? Take a moment to examine the data in this table.
Important to Know
Some substances smell only because they are contaminated with small molecules. For example, plastic itself does not have a smell, but it is often contaminated with small molecules that are used to make the plastic.
Many pieces of information can be gathered from the table. First, small molecules are gases at room temperature, medium-sized molecules are liquids, and large molecules are solids. One reason for this sorting by size is that large molecules tend to have more intermolecular attractions. This means that larger molecules are less likely to be gaseous and are less likely to get to your nose.
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
The grassy odor that is emitted when the lawn is mowed is cis-3-hexenal. This molecule is unstable and tends to rearrange to form trans-2-hexenal. Scientific evidence connects trans-2-hexenal with healing from stress.

Note that a molecule is considered medium-sized if it has about 5 to 19 carbon atoms and large if it has 20 or more carbon atoms. Carbon is a unique element because it bonds easily to other carbon atoms and forms four bonds. This makes it ideal for forming long and complex molecules.
In general, all medium-sized molecules have a smell, whether they are polar or nonpolar. The medium-sized molecules form a liquid because they are attracted to one another, but they are volatile, meaning they can go easily into the gas phase. Once these molecules get inside the nose, the intermolecular attractions between the molecules and the receptor sites allow you to detect a smell.
HEATING AND COOLING
What happens when you warm or cool molecular substances? Small molecules are already gases at room temperature. But larger molecules are liquids or solids. These molecules must undergo a phase change in order to be smelled. Warming a molecular liquid or solid will generally give it a more intense aroma. This is because heating a substance increases the rate at which a substance becomes a gas. Cooling a substance has the opposite effect.
237
When there are more molecules in the air, you are more likely to detect them. So the temperature of a substance will usually affect the strength of its smell. For example, you are more likely to smell warm food than cold leftovers from the fridge.
Example 2
Predict the Smell
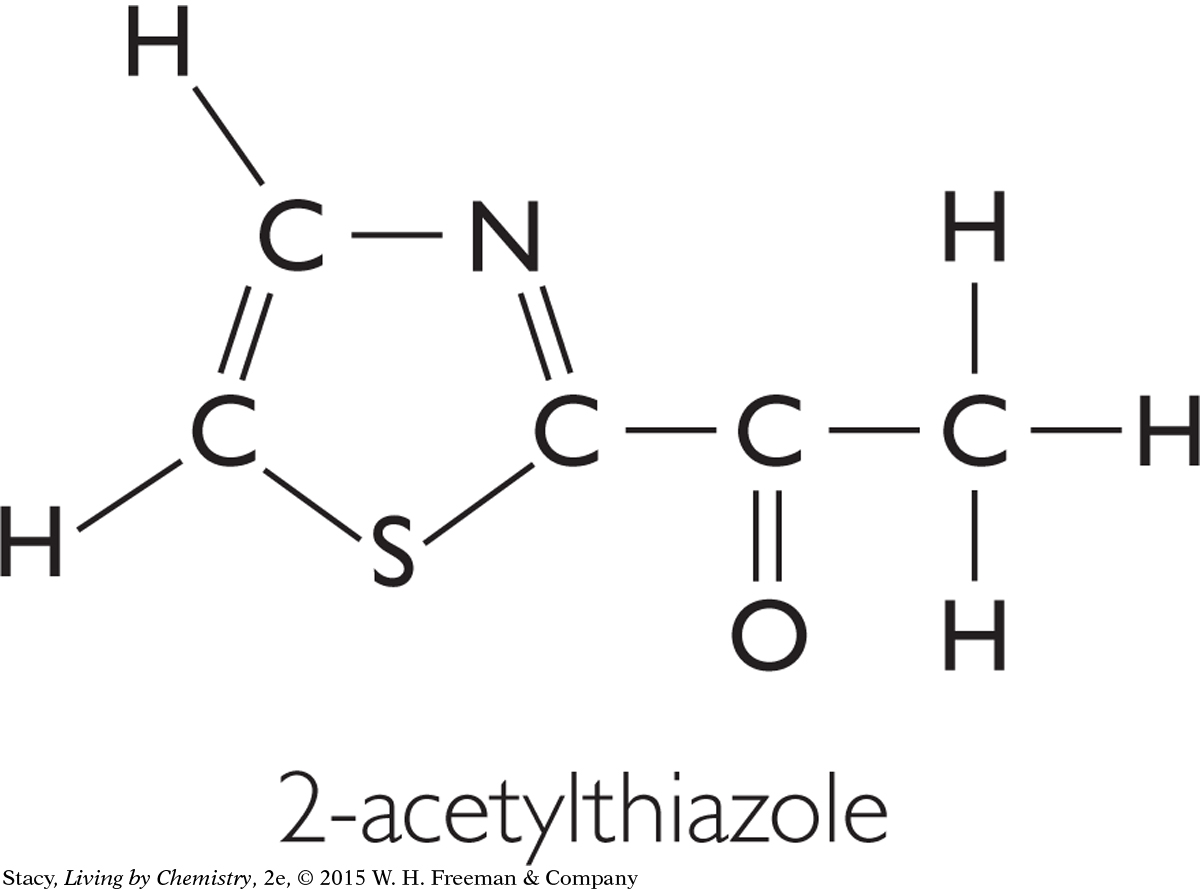
Below is the structural formula of a substance called 2-acetylthiazole. Predict whether this substance will have a smell at room temperature. Give evidence to support your answer.
Solution
This molecule’s formula is C5H5NSO. It has five carbon atoms. This is a medium-sized molecule, so it probably has some sort of smell. (In fact, it does. It is described as having a hazelnut or popcorn smell.)
LESSON SUMMARY
LESSON SUMMARY
What generalizations can you make about smell and molecules?
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
There is scientific evidence that some fish are better at smelling and tasting underwater than dogs are, out in the air. This explains why fish will often turn away from or avoid a fisherman’s lure. It is said that a salmon’s sense of smell can detect when a person puts their hand in the water 100 ft upstream.

Humans can smell some molecular substances. We cannot smell ionic, metallic, or network covalent substances. This is because the first requirement for a substance to be smelled is that it be in a gaseous form. The size of the molecule seems to be another important factor in predicting smell. Most medium-sized molecules will smell. Most large-sized molecules will not smell. Much of this is due to the effect of size on phase change. For small molecules, polarity also plays a role. Small nonpolar molecules do not have a smell. Small polar molecules do have a smell.
238
Exercises
Reading Questions
Explain what types of substances you would expect to have a smell and why.
Explain what types of substances you would expect not to have a smell and why.
Reason and Apply
Predict whether you would be able to smell these substances. Explain your reasoning.
decanol, C10H22O lead, Pb iron oxide, Fe2O3 potassium chloride, KCl Predict whether you would be able to smell these substances. Explain your reasoning.
neon, Ne ethane, C2H6 decane, C10H22 methylamine, CH5N If a substance is capable of becoming a gas under normal conditions, should you be able to smell it? Explain.
Molecules that have a structure containing more than about 20 carbon atoms rarely smell. Explain why this is so.
Your cotton T-shirt smells when you take it out of the clothes dryer. Explain why.
Occasionally you might go into a restaurant or a room that smells and after a while you stop noticing the odor. Explain what you think is going on.
From a biological point of view, why do you think it is useful for humans to be able to smell certain substances and not others?