Chapter 8 Summary
239

CHAPTER 8
Molecules in Action
SUMMARY
KEY TERMS
polar molecule
nonpolar molecule
partial charge
intermolecular force
electronegativity
dipole
diatomic molecule
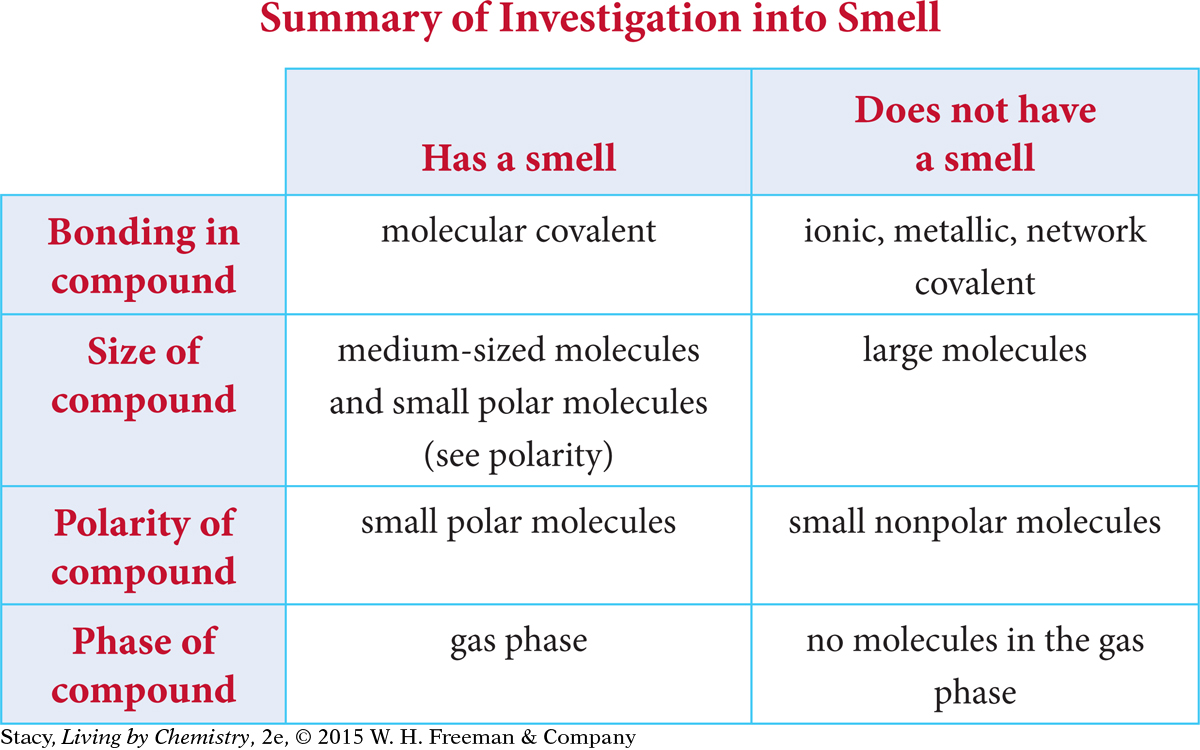
Smells Update
Some substances, like hot apple cider, have a smell. Other substances, like a granite tabletop, do not. The process of smelling is an interaction between molecules. To be smelled, a substance must become airborne and enter the nose. Once it is in the nose, it must dissolve and be detected. Receptor sites are composed of large, folded protein molecules with a polar area that attracts polar molecules.
REVIEW EXERCISES
Question 8.1
1. Which element is more electronegative?
Zn or Br
Li or Cs
Au or Al
Question 8.2
2. Place the following pairs of atoms in order of increasing bond polarity. Explain your reasoning.
| H–I | H–Cl | H–F |
Question 8.3
3. Draw the structural formula and place a partial positive and a partial negative charge on each atom in these molecules. Which molecules are polar? Explain your reasoning.
HCl
CH4
H2O
Question 8.4
4. At cold temperatures, hydrogen bromide, HBr, is a liquid.
Draw the Lewis dot structure for HBr. Label the dipole.
Would HBr be attracted to a charged wand? Would it form a round drop on waxed paper? Explain your reasoning.
In HBr, what type of bond exists between H and Br?
Do you expect HBr to have a smell? Explain.
Question 8.5
5. Use what you know about molecules to explain why you can’t smell perfume while the bottle is closed but can smell it once the bottle is opened.
Question 8.6
6. Which can you smell better, cold bread or warm bread right out of the oven? Explain why.