LESSON 47: Mirror, Mirror: Mirror-Image Isomers
242
THINK ABOUT IT

Limonene is a molecule that comes in two forms. The structural formulas for both forms are identical. Yet one smells like pine needles, while the other has the lemon-orange fragrance of citrus fruits. How can two molecules with the same molecular formula and the same structural formula have such distinct smells?

What are mirror-image isomers?
To answer this question, you will explore
Mirror Images of Molecules
Properties of Mirror-Image Isomers
Mirror Images of Molecules
EXPLORING THE TOPIC
Mirror Images of Molecules

Many things in nature are mirror images of each other: your ears, for example, or your eyebrows. While your ears may look physically identical, your left ear would not work well on the right side of your head. Your hands and feet are other good examples. Consider your left and right hands. They are made of the same parts and have the same overall structures. But if you try to place your left hand on your right hand with your palms down, the thumbs will point in opposite directions.
Your hands are mirror images of each other. That is, when you observe your left hand in a mirror, it will look identical to your right hand and vice versa. However, you cannot superimpose them on each other. No matter how you position your left hand, it is never identical to your right hand.
MOLECULES WITH A HANDEDNESS
Just as your right hand cannot be superimposed on your left hand, some molecules have a “handedness.”
The two limonene molecules described earlier have identical structures that are mirror images. The mirror images are not superimposable on one another. They are actually different molecules. You could say that one molecule is left-handed and the other is right-handed. They are isomers of one another.
Determining if two molecules are mirror images or the same molecule can be visually challenging. A three-dimensional model of the molecule and a mirror can help.
243
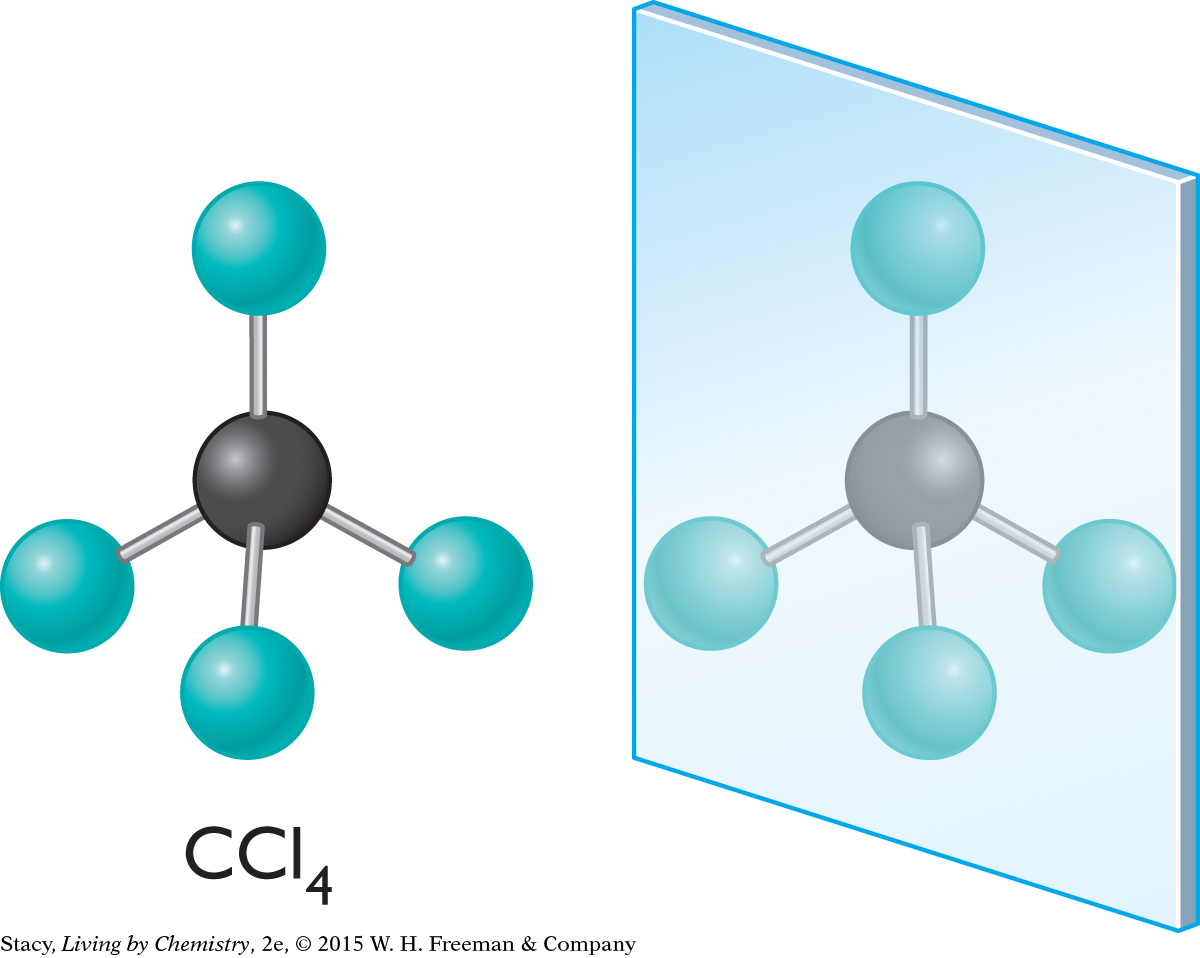
Consider the mirror image of a simple tetrahedral molecule, tetrachloromethane, CCl4. It is fairly easy to see that the mirror image can be superimposed on the original molecule. (In other words, you can imagine picking it up and turning or sliding it so that it matches the original.) So, tetrachloromethane does not have a mirror-image isomer. The molecule and its mirror image are identical.
Now consider dichloromethane, CCl2H2, and chlorofluoromethanol, CHClFOH. Take a moment to decide if either of the mirror images can be superimposed on its original molecule.
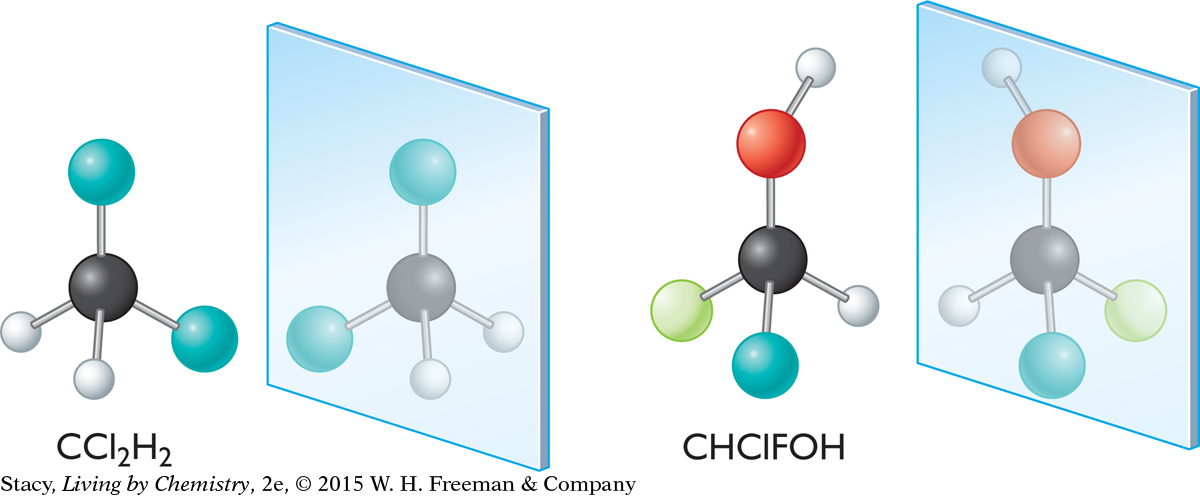
The mirror image of dichloromethane, CCl2H2, can be superimposed on the original molecule. However, when there are four different groups attached to a carbon atom as in the second molecule, the mirror image cannot be superimposed on the original molecule.
The two molecules of chlorofluoromethanol are mirror-image isomers. They have different properties.
Example
Objects in a Mirror
Which of the objects listed here look identical in a mirror? Which would look different? Explain any differences.
a. a can
|
b. a shoe
|
c. fluoromethane, CH3F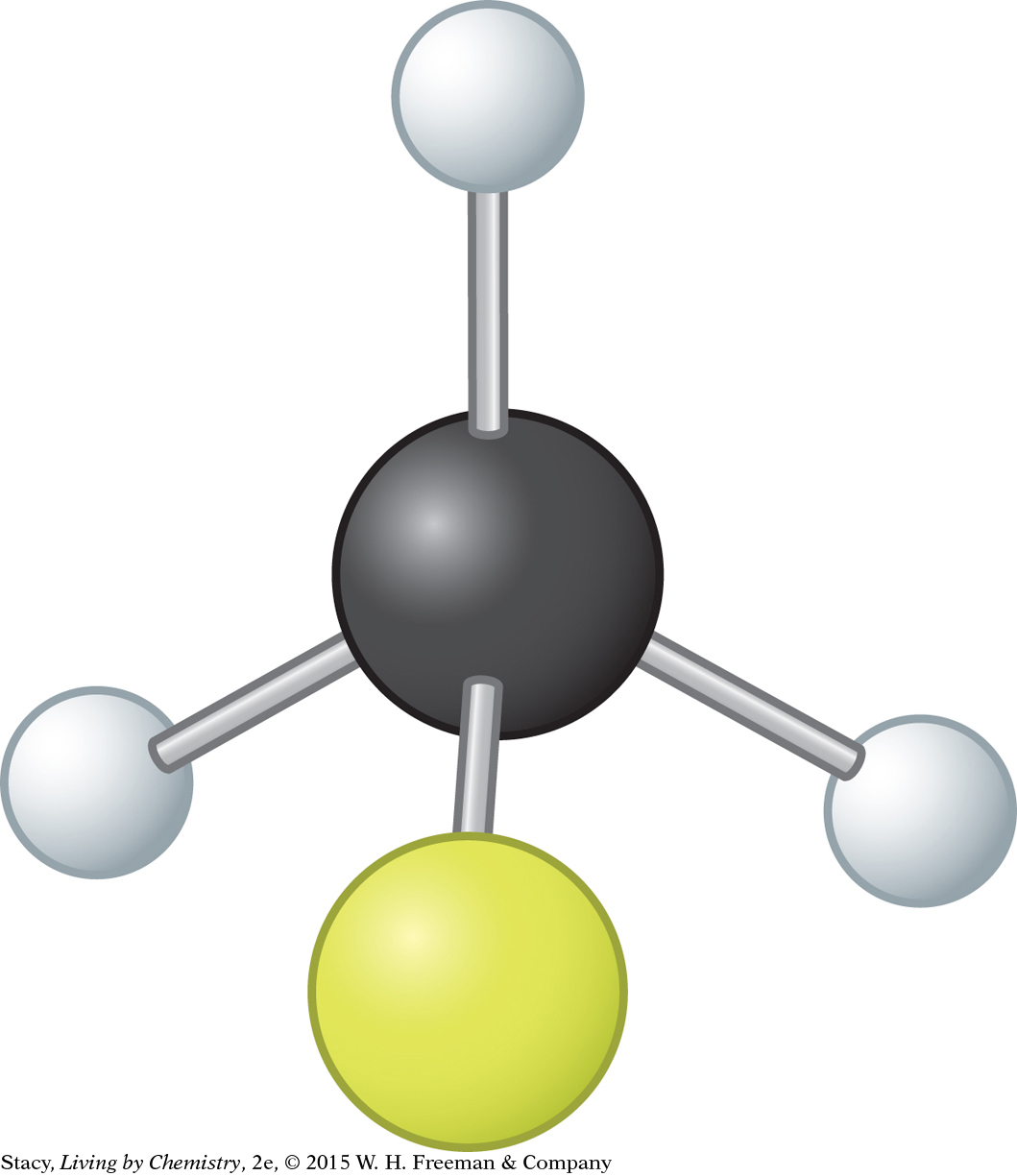
|
d. 2-chlorobutane, C4H9Cl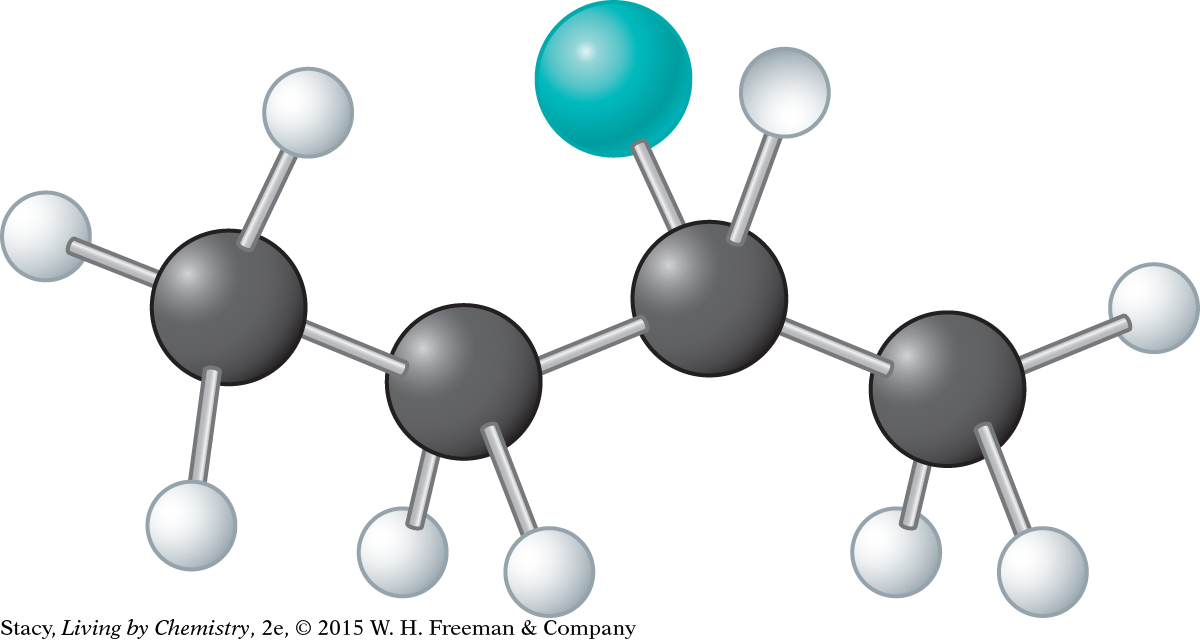
© D4Fish/iStockphoto (left), Pritmova Svetlana/Shutterstock (right)
|
Solution
244
A and C look identical in a mirror. B and D do not.
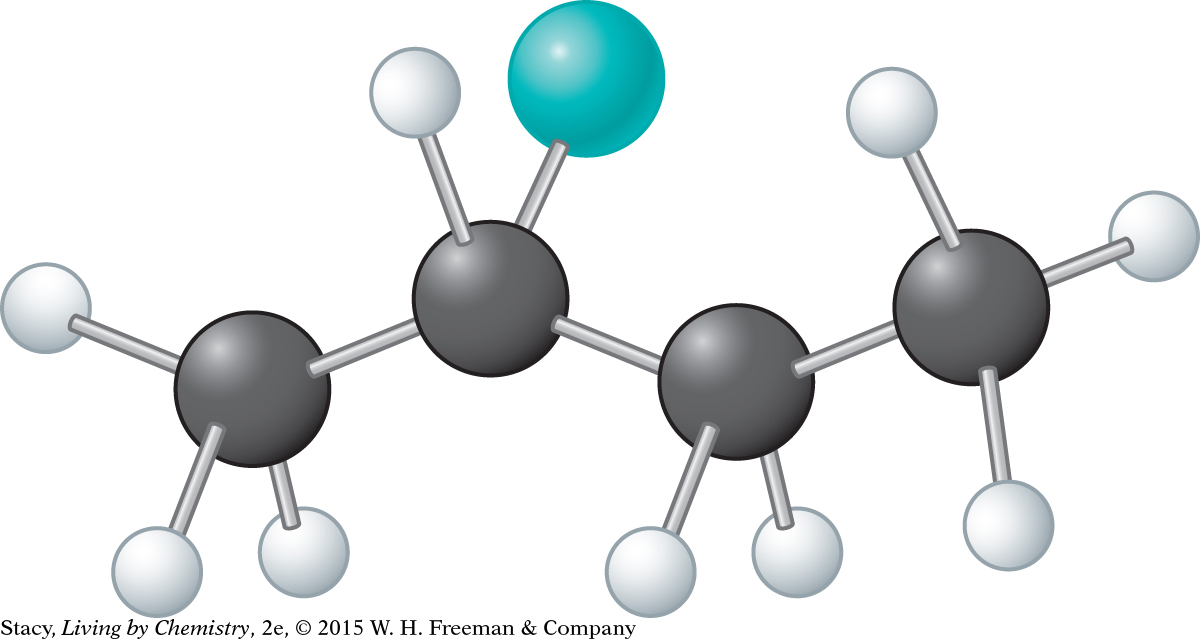
In the 2-chlorobutane molecule, the second carbon atom from the left has four different groups attached to it: CH3, H, Cl, and CH2CH3. Therefore, this molecule has a mirror-image isomer. When the H and Cl atoms of the two mirror-image isomers are in the same location, the CH3 and CH2CH3 groups are in opposite locations.
Properties of Mirror-Image Isomers
Properties of Mirror-Image Isomers
One way to figure out if a molecule has a mirror-image isomer is to check to see if any of the carbon atoms are attached to four different groups.
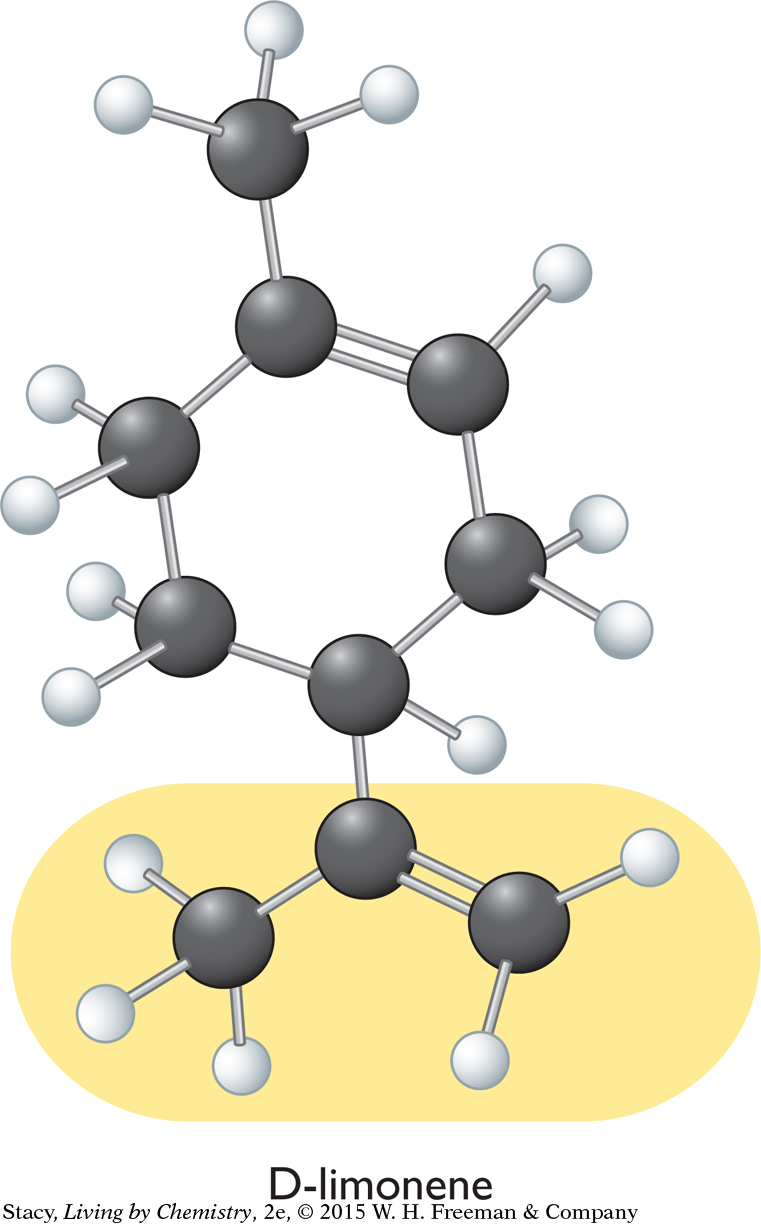
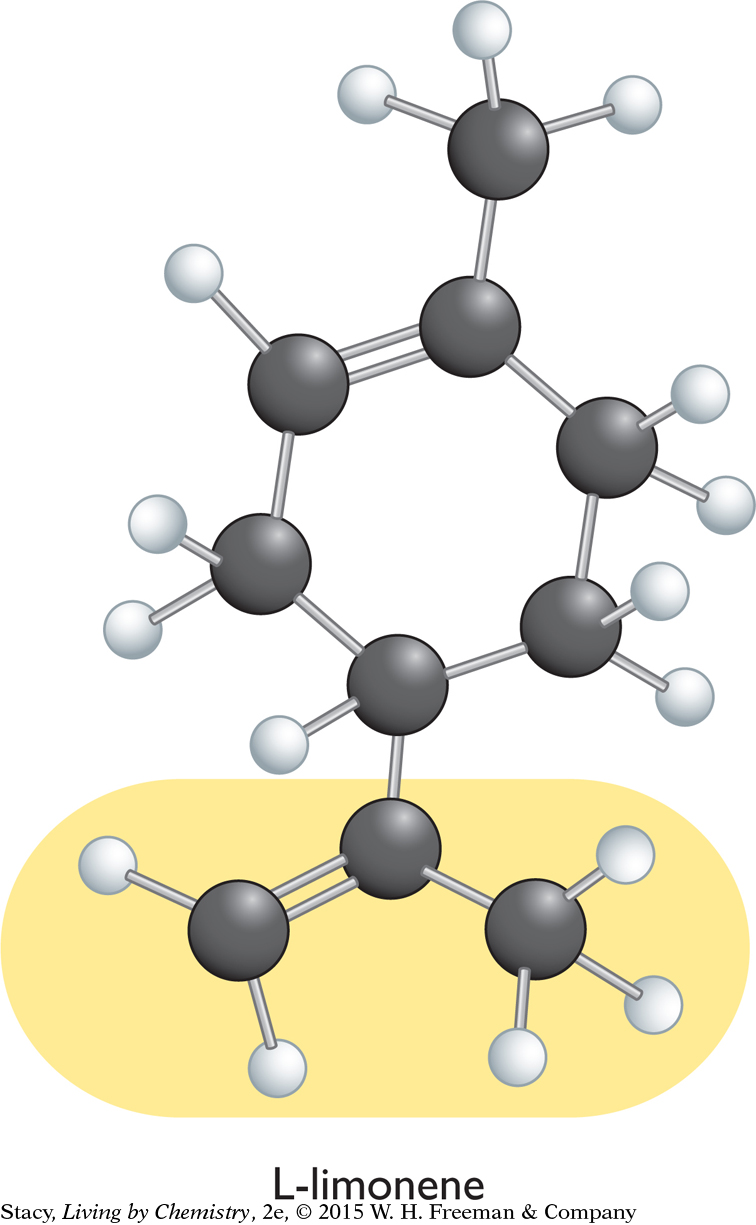
A structural formula for limonene, C10H16, is shown below (left). The illustration below (right) shows that nine of the carbon atoms in a limonene molecule are not attached to four different groups.
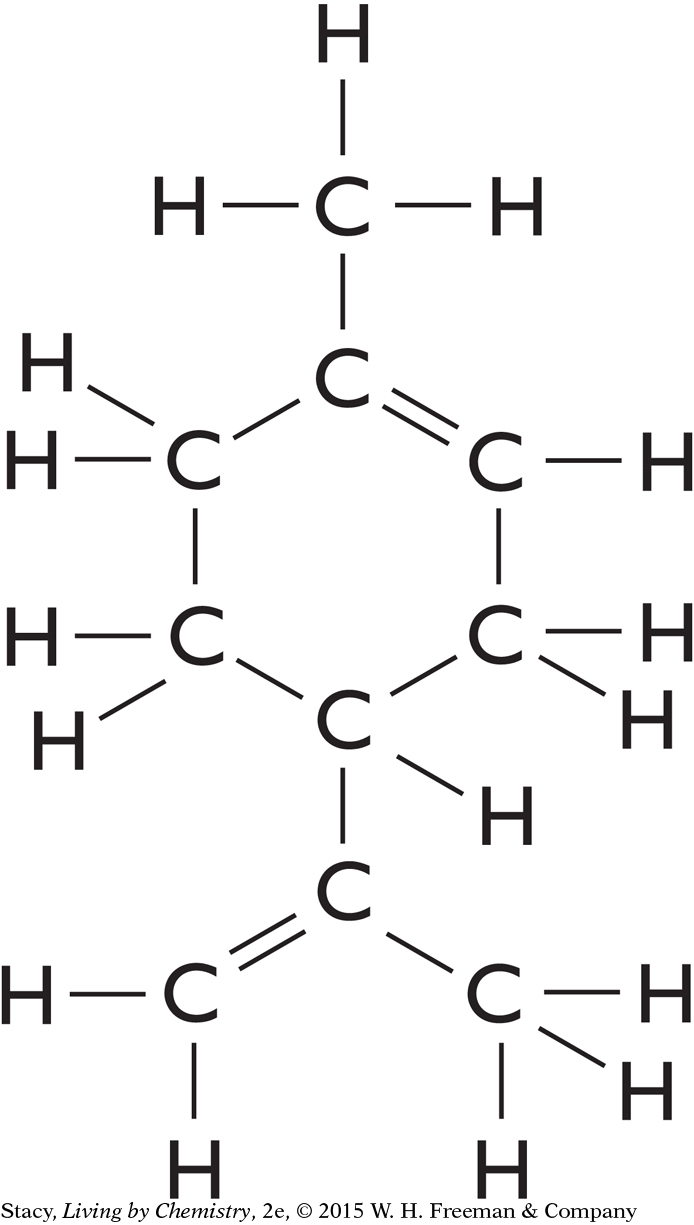
|
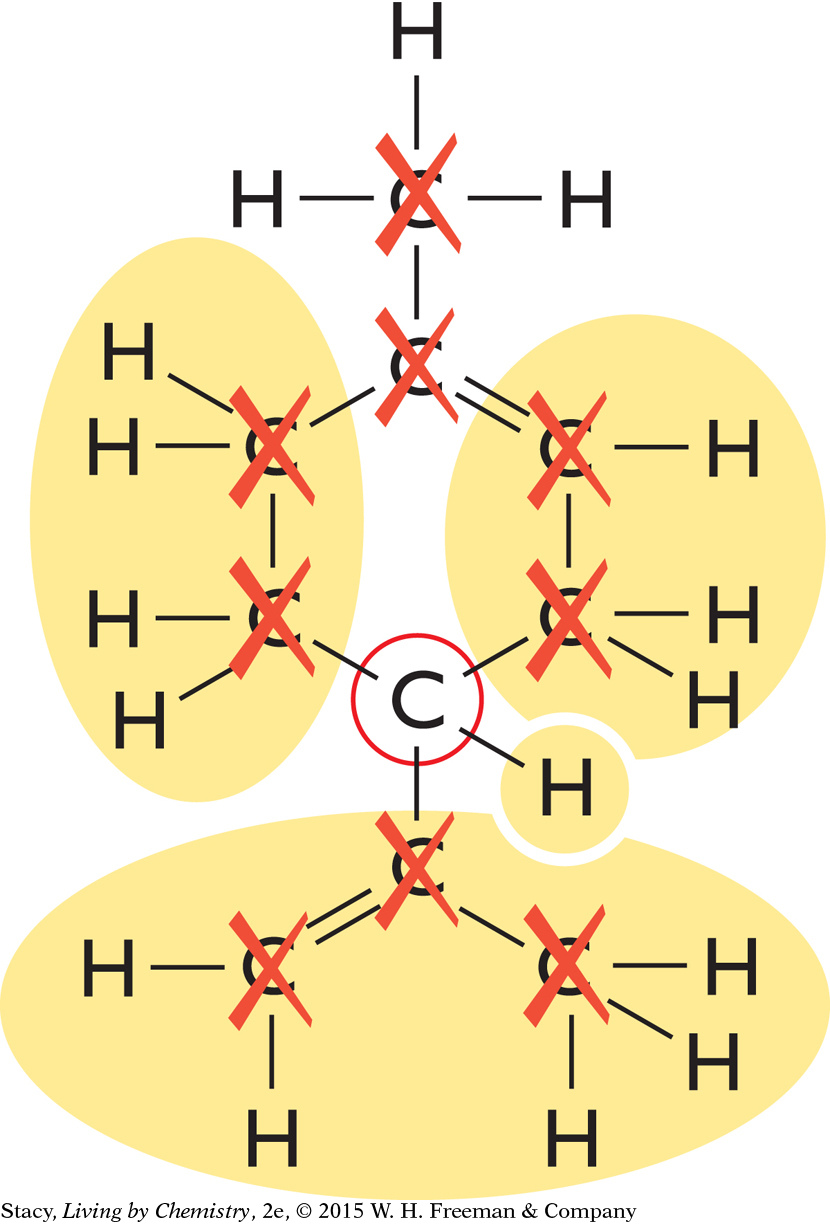
|
The remaining carbon atom, circled in the diagram, is attached to a hydrogen atom and three carbon “groups.” The two sides of the carbon ring are different from one another and considered different groups. You can conclude that limonene has a mirror-image isomer because it contains a carbon atom attached to four different groups of atoms. The two isomers are referred to as D-limonene and L-limonene.
The mirror-image isomers of limonene are shown on top of the next page. The main difference between them has to do with the position of the CCH2CH3 group highlighted in yellow. If you flip the L-limonene molecule around and try to superimpose it onto the D-limonene molecule, the CCH2CH3 group will point toward you in one isomer and away from you in the other isomer. It is as if you have aligned your thumbs on your right and left hands, only to find one palm down and the other palm up.
HEALTH CONNECTION
HEALTH
CONNECTION
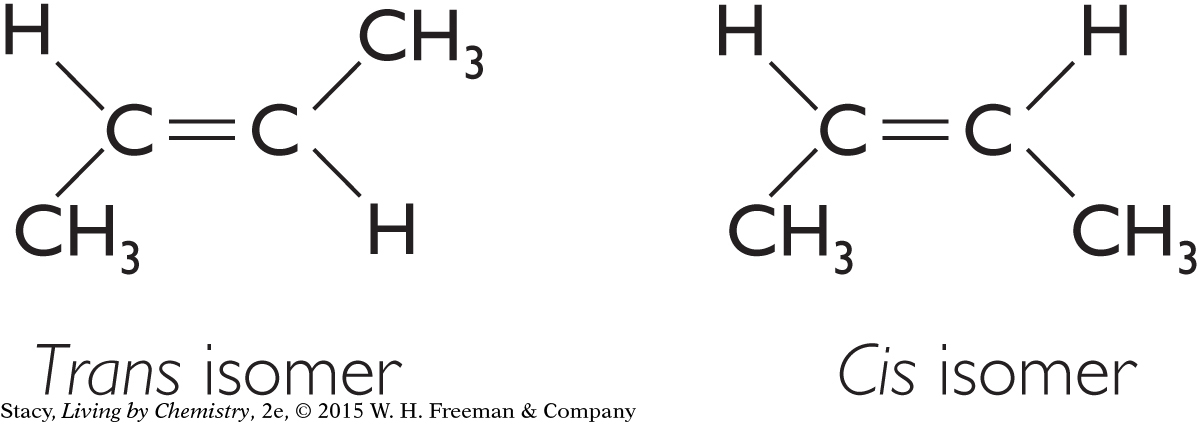
The two molecules below are isomers. The molecule with hydrogen atoms on opposite sides of the molecule is called a trans isomer. Most natural fatty acids are cis isomers. But when unsaturated fatty acids are hydrogenated industrially to make products like shortening, they often change into trans isomers. Research indicates these trans fats are linked to poor cardiovascular health and should be avoided in our diet.
245
|
|
DIFFERENT SMELLS

Just as your left foot does not fit into your right footprint, D-limonene does not fit into the smell receptor site for L-limonene.
Because the mirror-image isomers do not fit into the same receptor site, you would expect them to have distinct smells. Indeed, this is the case: D-limonene smells like citrus while L-limonene smells like pine. The two molecules have the same molecular formula, the same structural formula, and the same shape, but they are mirror images of one another.
LESSON SUMMARY
LESSON SUMMARY
What are mirror-image isomers?
KEY TERM
mirror-image isomer
Nature is full of mirror images that are not identical. Some objects and some molecules can be superimposed on their mirror images. For other objects, the mirror image is not superimposable. The mirror image cannot be positioned such that it is identical to the original object or molecule. Molecules with mirror images that cannot be superimposed are called mirror-image isomers. These molecules fit into different smell receptor sites and therefore have different smells.
Exercises
Reading Questions
What do you have to do to the mirror image of the letter “D” to superimpose the mirror image onto the original letter?
What types of molecules have mirror-image isomers?
246
Reason and Apply
List five objects that look different in a mirror and five that do not.
Draw mirror images of the capital letters in the English alphabet. Explain why symmetrical objects do not have mirror-image isomers.
Draw mirror images of the lowercase letters in the English alphabet. Which lowercase letters are mirror images of one another?
Draw the structural formulas for CH2O and ClHCO. Explain why these two molecules do not have mirror-image isomers.
Draw structural formulas for the molecules listed below. Draw the mirror image of each. Circle the molecules with mirror-image isomers.
CH4
CFH3
CClFH2
CBrClFH
Examine the molecules here. Which have mirror-image isomers? Explain your reasoning.
a. butyric acid 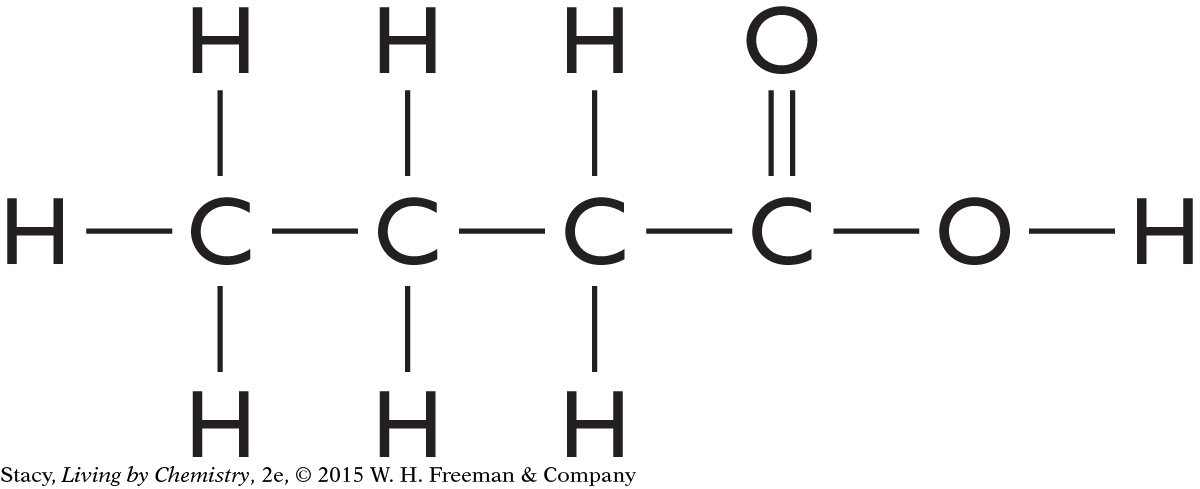
b. ethyl formate 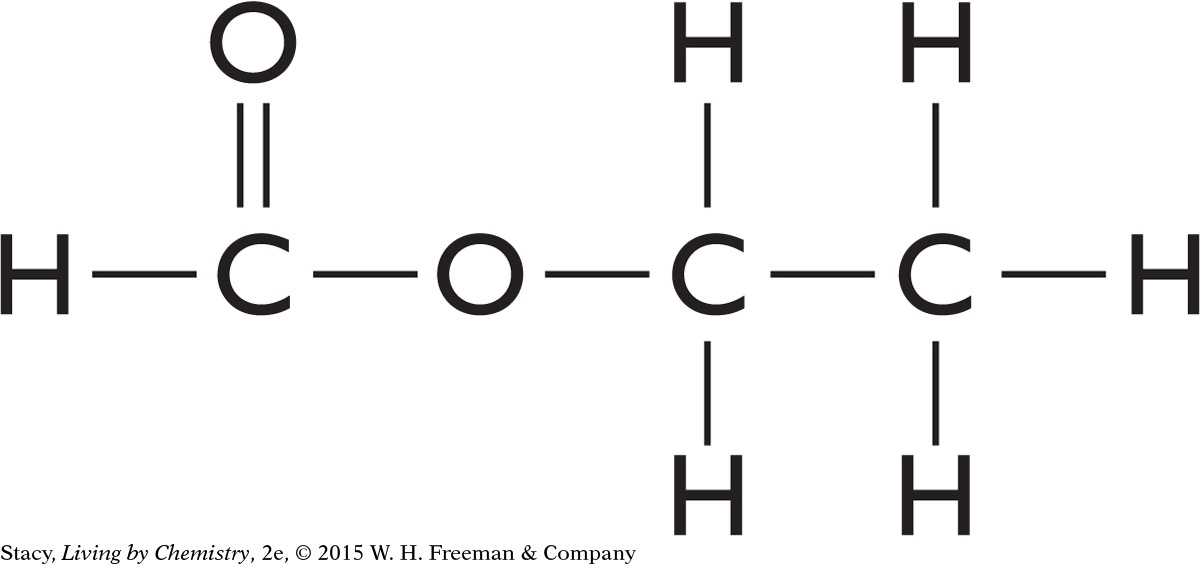
c. 2-butanol 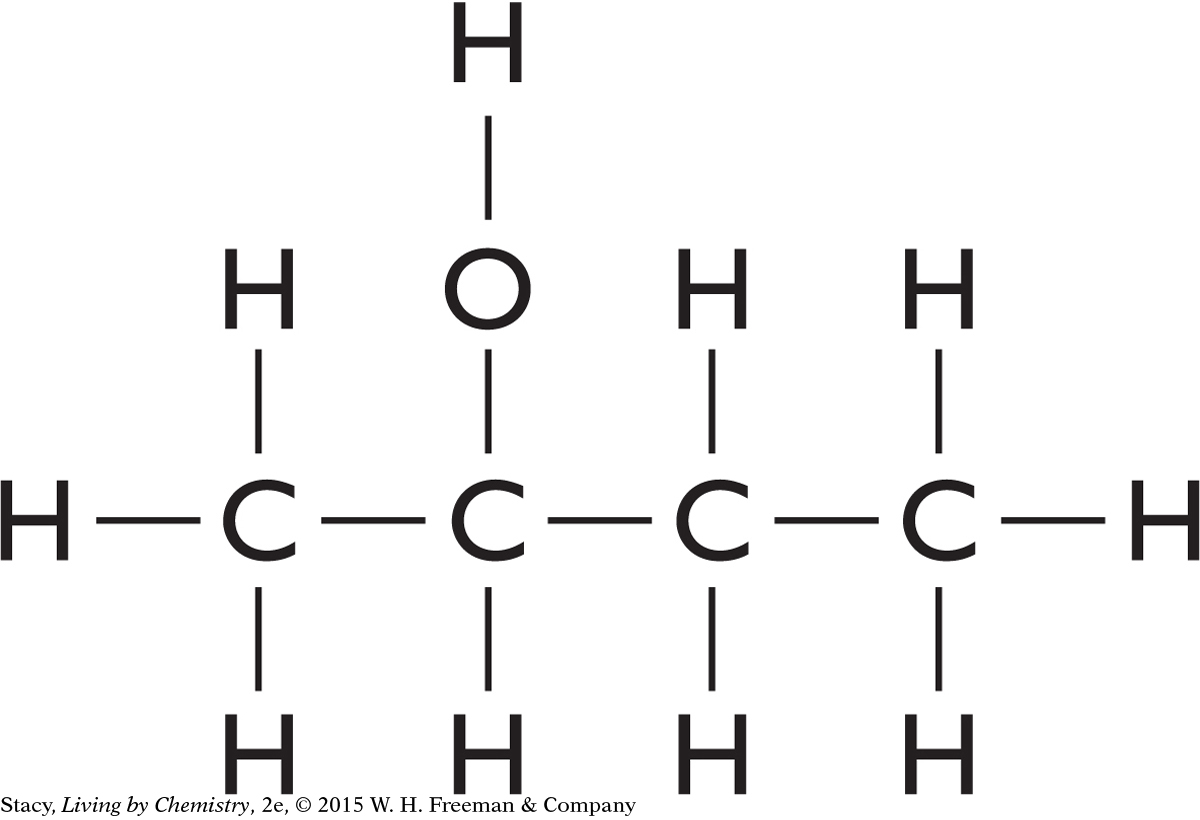
Two structural formulas are shown.
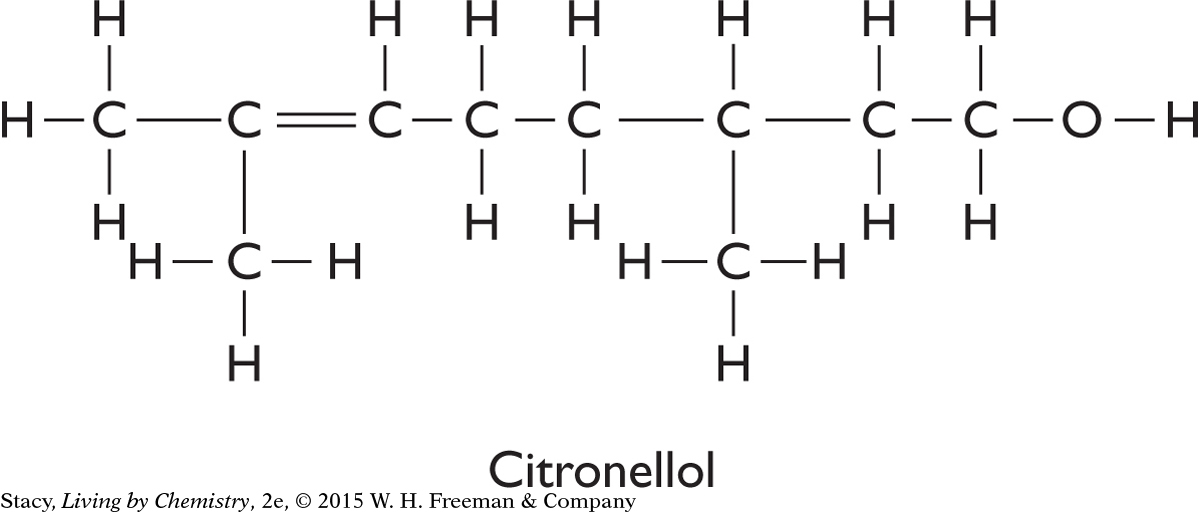
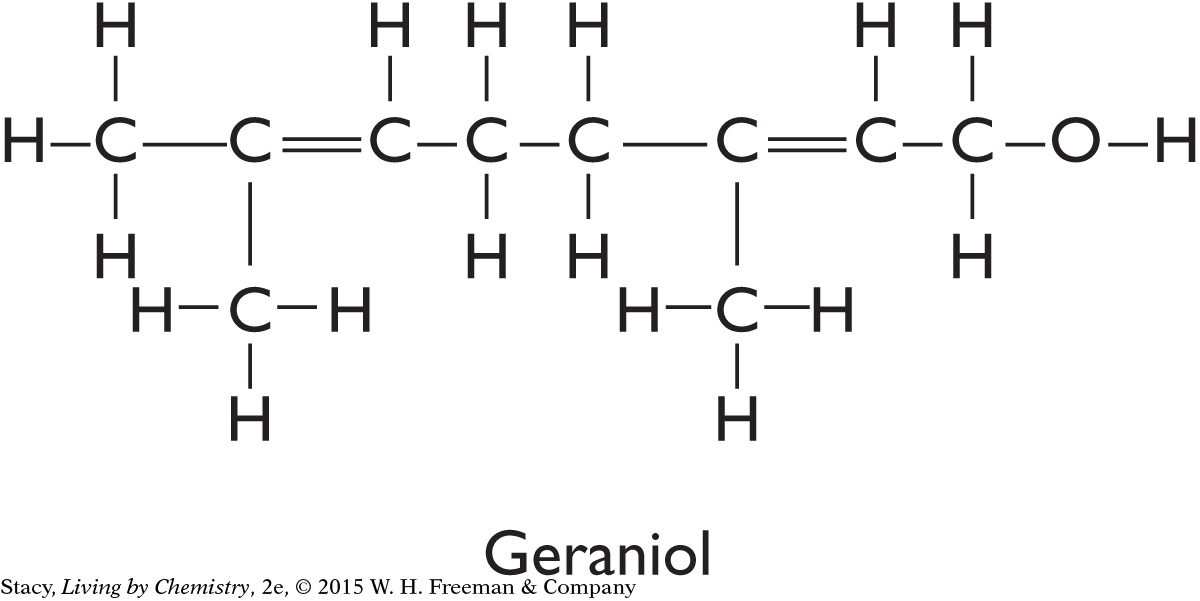
Write the molecular formulas for citronellol and geraniol.
Molecules with the structural formula for citronellol can smell either like insect repellant or like flowers. Explain why there are two distinct smells.
All molecules with the structural formula for geraniol smell like roses. Explain why there is only one smell for this molecule.