REVIEW EXERCISES
General Review
Write a brief and clear answer to each question. Be sure to show your work.
Question 1
1. What are isomers?
Question 2
2. Draw two isomers with the molecular formula C2H4O.
Question 3
3. A molecule can be described using a molecular formula, a structural formula, a ball-and-stick model, or a space-filling model. What information does each of these provide?
Question 4
4. Consider the structural formula for methyl salicylate. Name two functional groups that are in this molecule.
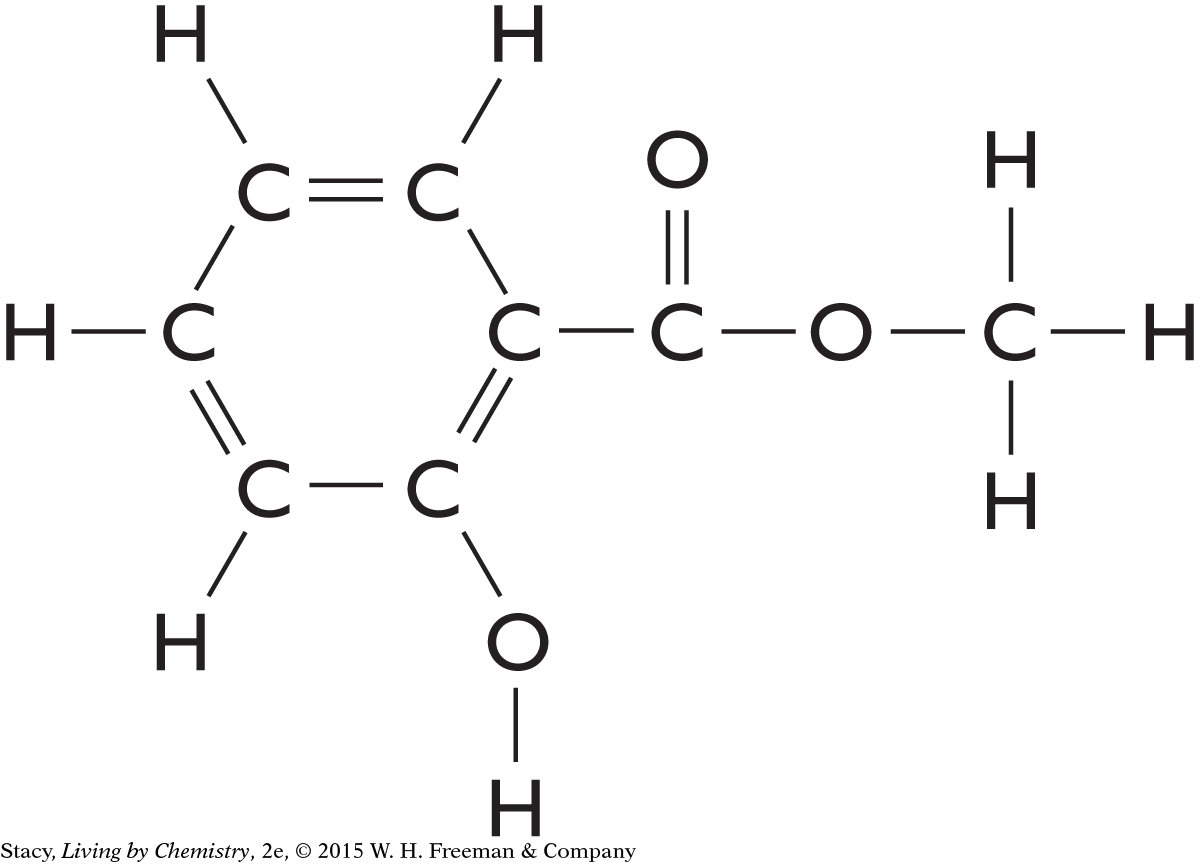
Question 5
5. Here is a ball-and-stick model of methyl salicylate.
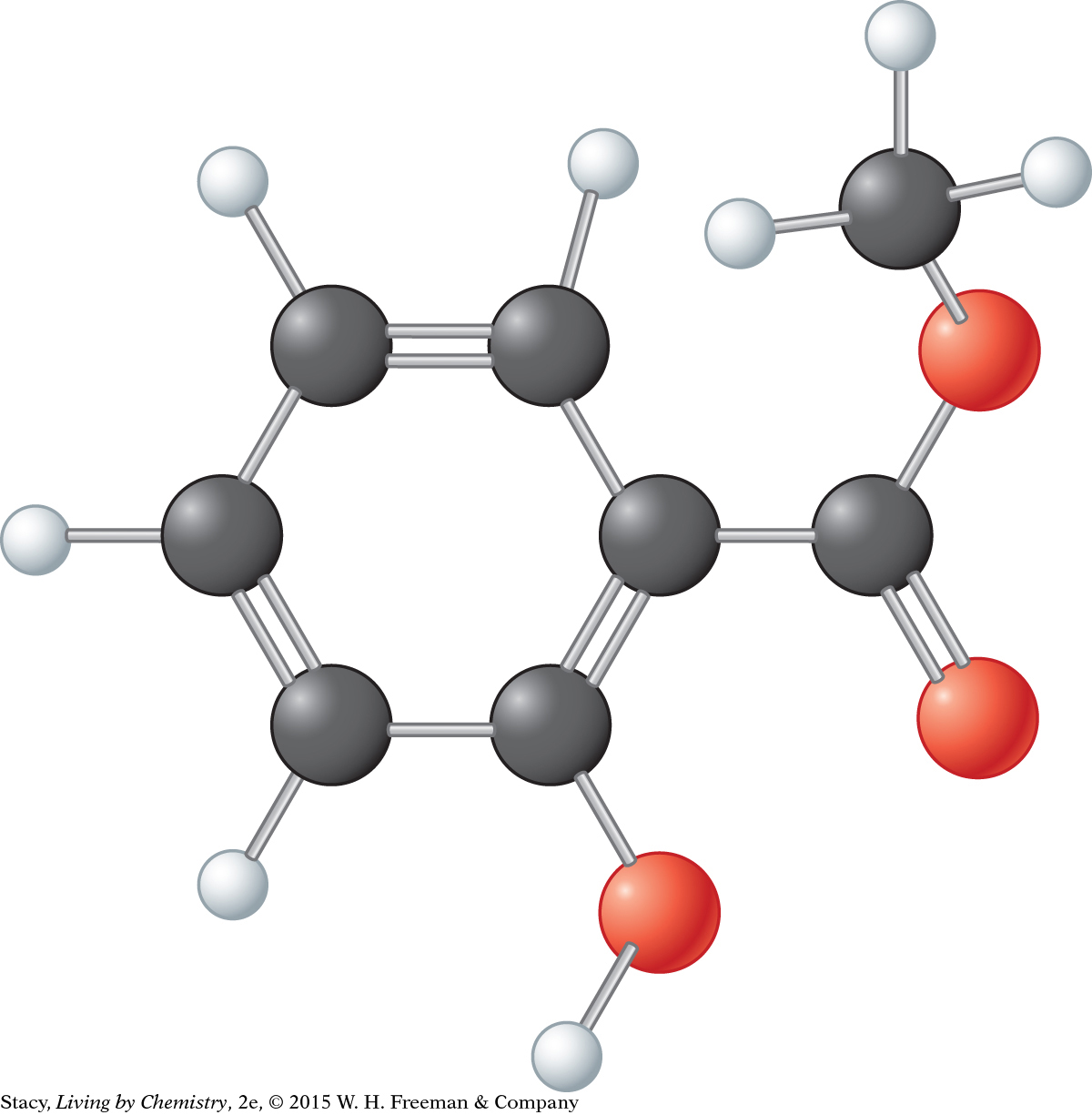
Does it follow the HONC 1234 rule?
Write the molecular formula for methyl salicylate.
Describe the overall shape of this molecule.
Find the oxygen atom that is bonded to two carbon atoms. Explain why this bond is bent.
Question 6
6. A polar molecule has the molecular formula C3H8O.
Draw the structural formula.
Draw the Lewis dot structure and label the shared electrons and the lone pairs.
Identify the dipoles.
Name the geometric shape around each carbon atom.
Discuss what the name of this compound might be. Explain your reasoning.
Question 7
7. What is a dipole? Explain why methane, CH4, has four dipoles but has no overall dipole.
Question 8
8. Table salt, NaCl, has no smell because
It is not made up of molecules.
It is an ionic compound.
No part of it enters the gas phase at room temperature.
All of the above.
None of the above.