10.3 Transport of mRNA Across the Nuclear Envelope
Fully processed mRNAs in the nucleus remain bound by hnRNP proteins in complexes referred to as nuclear mRNPs. Before an mRNA can be translated into its encoded protein, it must be exported from the nucleus into the cytoplasm. The nuclear envelope is a double membrane that separates the nucleus from the cytoplasm (see Figure 1-12). Like the plasma membrane surrounding a cell, each nuclear membrane consists of a water-impermeable phospholipid bilayer and multiple associated proteins. mRNPs and other macromolecules, including tRNAs and ribosomal subunits, traverse the nuclear envelope through nuclear pore complexes (NPCs). This section focuses on the export of mRNPs through NPCs and the mechanisms that allow some level of regulation of this step. Transport of mRNPs, proteins, and other cargoes through NPCs is discussed in greater detail in Chapter 13.
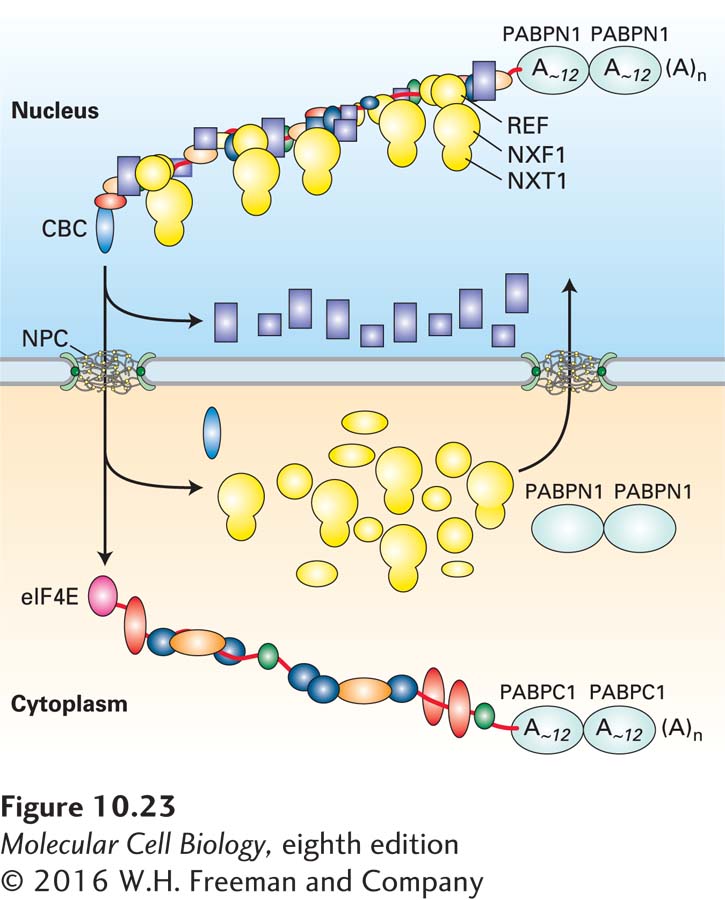
FIGURE 10-23 Remodeling of mRNPs during nuclear export. Some mRNP proteins (rectangles) dissociate from nuclear mRNP complexes before their export through an NPC. Others (ovals) are exported through the NPC with the mRNP, but dissociate from it in the cytoplasm and are shuttled back into the nucleus through an NPC. In the cytoplasm, translation initiation factor eIF4E replaces CBC bound to the 5′ cap, and PABPC1 replaces PABPN1.
Protein filaments extend from the core NPC scaffold into the nucleoplasm, forming an NPC nuclear basket (see Figure 10-23). Other protein filaments extend from the cytoplasmic face of the NPC into the cytoplasm. Both sets of filaments assist in mRNP export. Gle2, an adapter protein that reversibly binds both NXF1 and a protein in the nuclear basket, brings nuclear mRNPs to the NPC in preparation for export. A protein in the cytoplasmic filaments of the NPC binds an RNA helicase (Dbp5) that functions in the dissociation of NXF1/NXT1 and other hnRNP proteins from the mRNP as it reaches the cytoplasm.
In a process called mRNP remodeling, the proteins associated with an mRNA in the nuclear mRNP are exchanged for a different set of proteins as the mRNP is transported through the NPC (see Figure 10-23). Some nuclear mRNP proteins dissociate early in transport, remaining in the nucleus to bind to newly synthesized nascent pre-mRNA. Other nuclear mRNP proteins remain with the mRNP as it traverses the NPC and do not dissociate from the mRNP until the complex reaches the cytoplasm. Proteins in this category include the NXF1/NXT1 mRNP exporter, the nuclear cap-binding complex (CBC) bound to the 5′ cap, and PABPN1 bound to the poly(A) tail. These proteins dissociate from the mRNP on the cytoplasmic side of the NPC through the action of the Dbp5 RNA helicase that associates with the cytoplasmic NPC filaments, as discussed above. These proteins are then imported back into the nucleus, as described for other nuclear proteins in Chapter 13, where they can function in the export of another mRNP. In the cytoplasm, the cap-binding translation initiation factor eIF4E replaces the CBC bound to the 5′ cap of nuclear mRNPs (see Figure 5-23). In vertebrates, the nuclear poly(A)-binding protein PABPN1 is replaced with the cytoplasmic poly(A)-binding protein PABPC1 (so named to distinguish it from the nuclear PABPN1). Only a single PABP is found in budding yeast, in both the nucleus and the cytoplasm.
