Degradation of mRNAs in the Cytoplasm Occurs by Several Mechanisms
As mentioned above, the concentration of an mRNA is a function of both its rate of synthesis and its rate of degradation. For this reason, if two genes are transcribed at the same rate, the steady-state concentration of the corresponding mRNA that is more stable will be higher than the concentration of the other. The stability of an mRNA also determines how rapidly synthesis of the encoded protein can be shut down. For a stable mRNA, synthesis of the encoded protein persists long after transcription of the gene is repressed. Most bacterial mRNAs are unstable, decaying exponentially with a typical half-life of a few minutes. For this reason, a bacterial cell can rapidly adjust the synthesis of proteins to accommodate changes in the cellular environment. Most cells in multicellular organisms, on the other hand, exist in a fairly constant environment and carry out a specific set of functions over days to months or even the lifetime of the organism (neurons, for example). Accordingly, most mRNAs of higher eukaryotes have half-lives of many hours.
However, some proteins in eukaryotic cells are required only for short periods and must be expressed in bursts. For example, as discussed above, certain signaling molecules called cytokines, which are involved in regulating the immune response of mammals, are synthesized and secreted in short bursts (see Chapter 23). Similarly, many of the transcription factors that regulate the onset of the S phase of the cell cycle, such as Fos and Jun, are synthesized only for brief periods (see Chapter 19). The expression of such proteins occurs in short bursts because transcription of their genes can be rapidly turned on and off, and their mRNAs have unusually short half-lives, on the order of 30 minutes or less.
Cytoplasmic mRNAs are degraded by one of the three pathways shown in Figure 10-27. For most mRNAs, the deadenylation-dependent pathway is followed: the length of the poly(A) tail gradually decreases with time through the action of a deadenylating nuclease complex. When the tail has been shortened sufficiently, PABPC1 molecules can no longer bind to it and stabilize the interaction of the 5′ cap and translation initiation factors (see Figure 5-23, which summarizes the steps of translation initiation). The exposed cap is then removed by a decapping enzyme (DCP1/DCP2), leaving the unprotected mRNA susceptible to degradation by XRN1, a 5′→3′ exoribonuclease. Removal of the poly(A) tail also makes mRNAs susceptible to degradation by cytoplasmic exosomes containing 3′→5′ exonucleases. The 5′→3′ exonuclease pathway predominates in yeast, and the 3′→5′ exosome pathway predominates in mammalian cells. The decapping enzymes and 5′→3′ exonuclease are concentrated in P bodies (processing bodies, described below), regions of the cytoplasm with unusually high concentrations of RNPs.
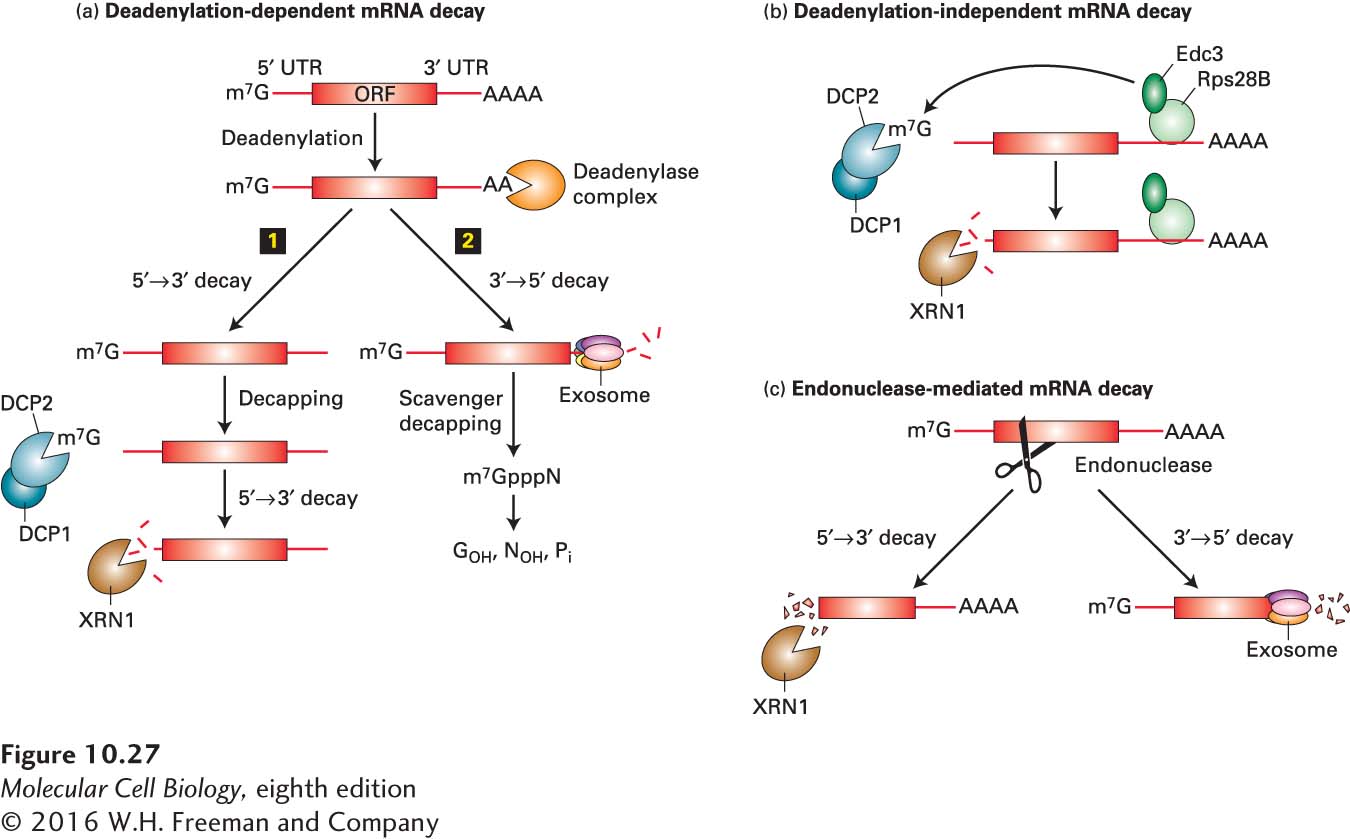
FIGURE 10-27 Pathways for degradation Of eukaryotic mRNAS. (a) In the most common pathway of mRNA degradation, the deadenylation-dependent pathway, the poly(A) tail is progressively shortened by a deadenylase complex until it reaches a length of 20 or fewer A residues, at which point the interaction between PABPC1 and the remaining poly(A) is destabilized, leading to weakened interactions between the 5′ cap and translation initiation factors (see Figure 5-23). The deadenylated mRNA then may either (1) be decapped by the DCP1/DCP2 deadenylation complex and degraded by XRN1, a 5′→3′ exonuclease, or (2) be degraded by 3′→5′ exonucleases in cytoplasmic exosomes. (b) Other mRNAs are decapped before they are deadenylated and then degraded by the XRN1 5′→3′ exonuclease. In the example shown from yeast, an RNA-binding protein Rps28B binds a sequence in the 3’-UTR of its own mRNA, which then interacts with Edc3 (enhancer of decapping 3). Edc3 then recruits the DCP1/2 decapping enzyme to the mRNA, auto regulating expression of Rps28B. (c) Some mRNAs are cleaved internally by an endonuclease and the fragments degraded by a cytoplasmic exosome and the XRN1 exonuclease. See N. L. Garneau, J. Wilusz, and C. J. Wilusz, 2007, Nat. Rev. Mol. Cell Biol. 8:113.
Some mRNAs are degraded primarily by a deadenylation-independent decapping pathway (Figure 10-27b). Certain sequences at the 5′ end of an mRNA make the cap sensitive to the decapping enzyme. For these mRNAs, the rate at which they are decapped controls the rate at which they are degraded because once the 5′ cap is removed, the RNA is rapidly hydrolyzed by the 5′→3′ exoribonuclease XRN1.
Other mRNAs are degraded by an endonucleolytic pathway that does not involve decapping or significant deadenylation (Figure 10-27c). One example of this type of pathway is the RNA interference pathway discussed below. Each siRNA-RISC complex can degrade thousands of targeted RNA molecules. The fragments generated by internal cleavage are then degraded by exonucleases.
The rate of mRNA deadenylation varies inversely with the frequency of translation initiation for an mRNA: the higher the frequency of initiation, the slower the rate of deadenylation. This relationship is probably due to the reciprocal interactions between translation initiation factors bound at the 5′ cap and PABPC1 bound to the poly(A) tail. For an mRNA that is translated at a high rate, initiation factors are bound to the cap much of the time, stabilizing the binding of PABPC1 and thereby protecting the poly(A) tail from deadenylating nuclease complexes.
Many short-lived mRNAs in mammalian cells—those encoding proteins such as cytokines and transcription factors whose concentrations must change rapidly—contain multiple, sometimes overlapping copies of the sequence AUUUA in their 3′ untranslated region. These sequences are known as AU-rich elements. Specific RNA-binding proteins have been found that bind to these 3′ AU-rich sequences and also interact with a deadenylating enzyme and with the exosome, causing rapid deadenylation and subsequent 3′→5′ degradation of these mRNAs. This mechanism uncouples the rate of mRNA degradation from the frequency of translation. Thus mRNAs containing AU-rich elements can be translated at high frequency yet can also be degraded rapidly, allowing the encoded proteins to be expressed in short bursts.
P bodies are dense cytoplasmic domains many times the size of a ribosome. They are sites of translational repression that contain no ribosomes or translation factors. They are also major sites of mRNA degradation in the cytoplasm. These dense regions of cytoplasm contain the decapping enzyme (DCP1/DCP2), activators of decapping (DHH, PAT1, LSM1-7), and the major 5′→3′ exoribonuclease XRN1, as well as densely associated mRNAs. P bodies are dynamic structures that grow and shrink in size depending on the rate at which mRNPs associate with them, the rate at which mRNAs are degraded, and the rate at which mRNPs exit P bodies and reenter the pool of translated mRNPs. Those mRNAs whose translation is inhibited by imperfect base pairing of miRNAs are major components of P bodies, as we will see shortly.
