Micro-RNAs Repress Translation and Induce Degradation of Specific mRNAs
Micro-RNAs (miRNAs) were first discovered during analysis of mutations in the lin-4 and let-7 genes of the nematode C. elegans, which influence the development of that organism. Cloning and analysis of wild-type lin-4 and let-7 revealed that they do not encode protein products, but rather RNAs only 21 and 22 nucleotides long, respectively. These RNAs hybridize to the 3′ untranslated regions (3′ UTRs) of specific target mRNAs. For example, the lin-4 miRNA, which is expressed early in embryogenesis, hybridizes to the 3′ UTRs of both the lin-14 and lin-28 mRNAs in the cytoplasm, thereby repressing their translation. Expression of lin-4 miRNA ceases later in development, allowing the translation of newly synthesized lin-14 and lin-28 mRNAs at that time. Expression of let-7 miRNA occurs at comparable times during embryogenesis in all bilaterally symmetric animals.
Regulation of translation by miRNAs appears to be widespread in all multicellular plants and animals. In the past few years, small RNAs of 20–26 nucleotides have been isolated, cloned, and sequenced from various tissues of multiple model organisms. Recent estimates suggest that the expression of one-third of all human genes is regulated by the roughly 1900 human miRNAs isolated from various tissues. The potential for regulation of multiple mRNAs by one miRNA is great because base pairing between the miRNAs and the 3′ ends of the mRNAs that they regulate need not be perfect (Figure 10-28). In fact, considerable experimentation with synthetic miRNAs has shown that complementarity between bases 2–7 at the 5′ end of an miRNA (called the “seed” sequence) and its target-mRNA 3′ UTR is most critical for target-mRNA selection.
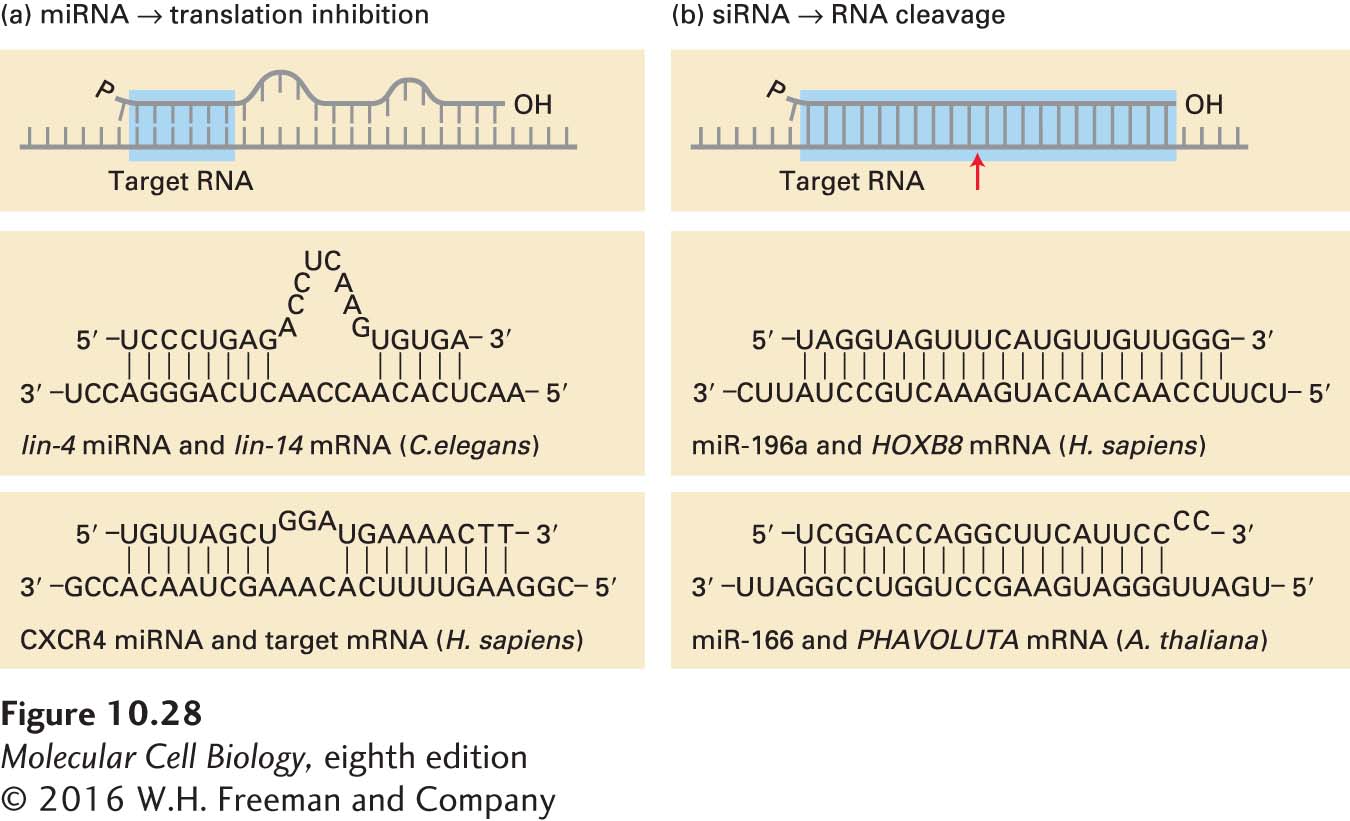
FIGURE 10-28 Base pairing with target RNAs distinguishes miRNA and siRNA. (a) miRNAs hybridize imperfectly with their target mRNAs, repressing translation of the mRNA. Nucleotides 2–7 of an miRNA (highlighted blue) are the most critical for targeting it to a specific mRNA. The CXCR4 miRNA shown at the bottom is a synthetic oligonucleotide introduced into cells by transfection. (b) siRNA hybridizes perfectly with its target mRNA, causing cleavage of the mRNA at the position indicated by the red arrow, triggering its rapid degradation. See P. D. Zamore and B. Haley, 2005, Science 309:1519.
Most miRNAs are processed from RNA polymerase II transcripts that are several hundred to thousands of nucleotides in length, called pri-miRNAs (for primary transcript) (Figure 10-29). A pri-miRNA can contain the sequence of one or more miRNAs. Some miRNAs are also processed from excised introns and from 3′ UTRs of some pre-mRNAs. Within these long transcripts are sequences that fold into hairpin structures about 70 nucleotides in length with imperfect base pairing in the stem. A nuclear RNase specific for double-stranded RNA, called Drosha, acts with a nuclear double-stranded RNA–binding protein, called DGCR8 (DiGeorge syndrome chromosomal region 8, named for its association with this genetic disease) in humans (Pasha in Drosophila) to cleave the hairpin region out of the long precursor RNA, generating a pre-miRNA. Pre-miRNAs are recognized and bound by a specific nuclear export factor, exportin 5, which allows them to diffuse through the inner channel of the nuclear pore complex. Once it reaches the cytoplasm, a cytoplasmic double-stranded RNA–specific RNase, called Dicer, acts with a cytoplasmic double-stranded RNA–binding protein, called TRBP in humans (for Tar binding protein; called Loquacious in Drosophila), to further process the pre-miRNA into a double-stranded miRNA. The double-stranded miRNA is approximately two turns of an A-form RNA helix in length, with strands 21–23 nucleotides long and two unpaired 3′ nucleotides at each end. Finally, one of the two strands is selected for assembly into a mature RNA-induced silencing complex (RISC), which contains a single-stranded mature miRNA bound by a multidomain Argonaute protein, a member of a protein family with a recognizable conserved sequence, as well as additional proteins. Several Argonaute proteins are expressed in some organisms, especially plants, and are found in distinct RISC complexes with different functions. Humans express four Argonaute proteins. AGO2 is the human Argonaute protein in miRNA-containing RISC complexes. The other human Argonaute proteins have partially overlapping functions because knockout of all four human Argonaute proteins is lethal to human embryonic stem cells, but any one of the four is sufficient for viability. The specific functions of the other Argonaute proteins during mouse development are currently under study.
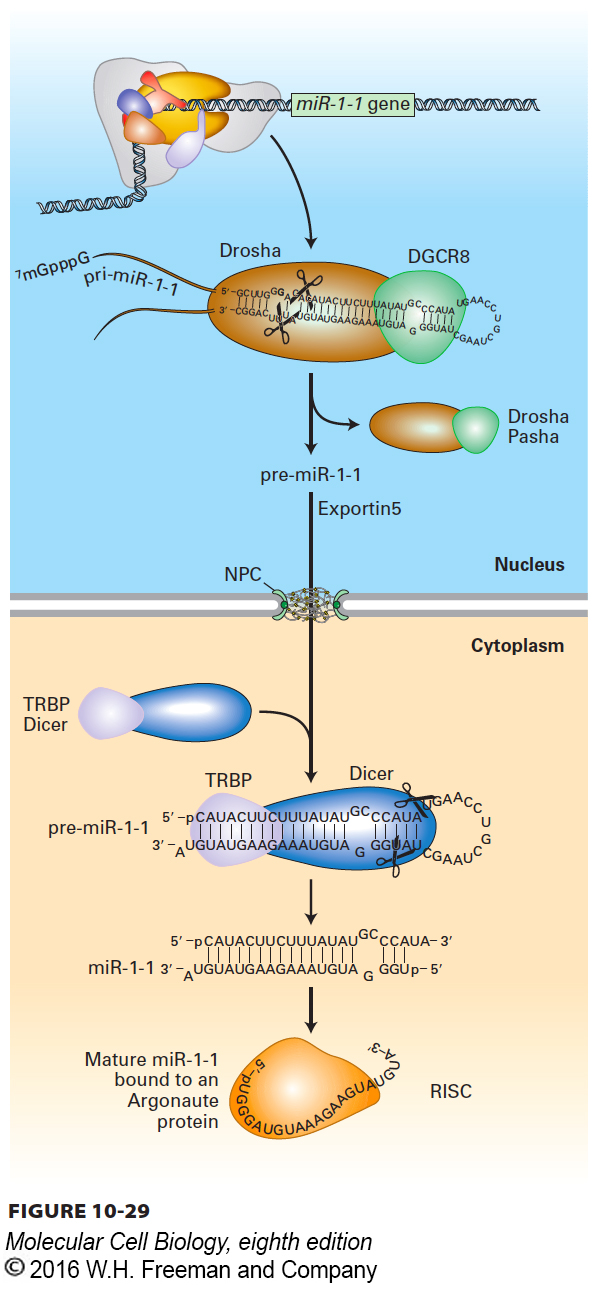
FIGURE 10-29 Processing of miRNA. This diagram shows transcription and processing of the miR-1-1 miRNA. The primary miRNA transcript (pri-miRNA) is transcribed by RNA polymerase II. The nuclear double-stranded RNA–specific endoribonuclease Drosha, with its partner, double-stranded RNA–binding protein DGCR8 (Pasha in Drosophila), makes the initial cleavages in the pri-miRNA, generating a ~70-nucleotide pre-miRNA that is exported to the cytoplasm by nuclear transporter exportin 5. The pre-miRNA is further processed in the cytoplasm by Dicer, in conjunction with the double-stranded RNA–binding protein TRBP (Loquacious in Drosophila), into a double-stranded miRNA with two-base single-stranded 3′ ends. Finally, one of the two strands is incorporated into a RISC complex, where it is bound by an Argonaute protein. See P. D. Zamore and B. Haley, 2005, Science 309:1519.
The miRNA-RISC complexes associate with target mRNPs by base pairing between the Argonaute-bound mature miRNA and complementary regions in the 3′ UTRs of target mRNAs (see Figure 10-28). Inhibition of target-mRNA translation requires the binding of two or more RISC complexes to distinct complementary regions in the target-mRNA 3′ UTR. Generally the more RISC complexes bound to the 3′ UTR of an mRNA, the greater the repression of translation. This mechanism allows combinatorial regulation of mRNA translation by separately regulating the transcription of two or more different pri-miRNAs, which are processed into miRNAs required in combination to suppress the translation of a specific target mRNA.
The mechanism by which the binding of several RISC complexes to an mRNA inhibits translation initiation is currently being analyzed. Binding of RISC complexes causes the bound mRNPs to associate with P bodies. Since P bodies are major sites of mRNA degradation where the decapping complex DPC1/DPC2, the 5′→3′ exonuclease XRN1, and cytoplasmic exosomes are concentrated, mRNAs bound by several RISC complexes are degraded.
As mentioned earlier, approximately 1900 different human miRNAs have been observed, most of which are expressed only in specific cell types at particular times during embryogenesis and after birth. Determining the function of these miRNAs is currently a highly active area of research. In one example, a specific miRNA, called miR-133, is induced when myoblasts differentiate into muscle cells. This miRNA suppresses the translation of PTB, a regulatory splicing factor that functions similarly to Sxl in Drosophila (see Figure 10-18). PTB binds to 3′ splice sites in the pre-mRNAs of many genes, leading to exon skipping or use of alternative 3′ splice sites. When miR-133 is expressed in differentiating myoblasts, the PTB concentration falls. As a result, alternative isoforms of multiple proteins important for muscle-cell function are expressed in the differentiated cells.
Other examples of miRNA regulation are being discovered at a rapid pace in various organisms. Knocking out the dicer gene eliminates the generation of miRNAs in mammals. This manipulation causes embryonic death early in mouse development. When dicer is knocked out only in limb primordia, however, the influence of miRNAs on the development of the nonessential limbs can be observed (Figure 10-30). Although all major cell types in the limb differentiate and the fundamental aspects of limb patterning are maintained, development is abnormal—demonstrating the importance of miRNAs in regulating the proper level of translation of multiple mRNAs. In effect, miRNAs “fine-tune” gene expression to the appropriate level for gene function in various cell types. Of the 1900 human miRNAs, 53 appear to be unique to primates. It seems likely that new miRNAs have arisen readily during evolution by the duplication of a pri-miRNA gene followed by mutation of bases encoding the mature miRNA. miRNAs are particularly abundant in plants—more than 1.5 million distinct miRNAs have been characterized in Arabidopsis thaliana!
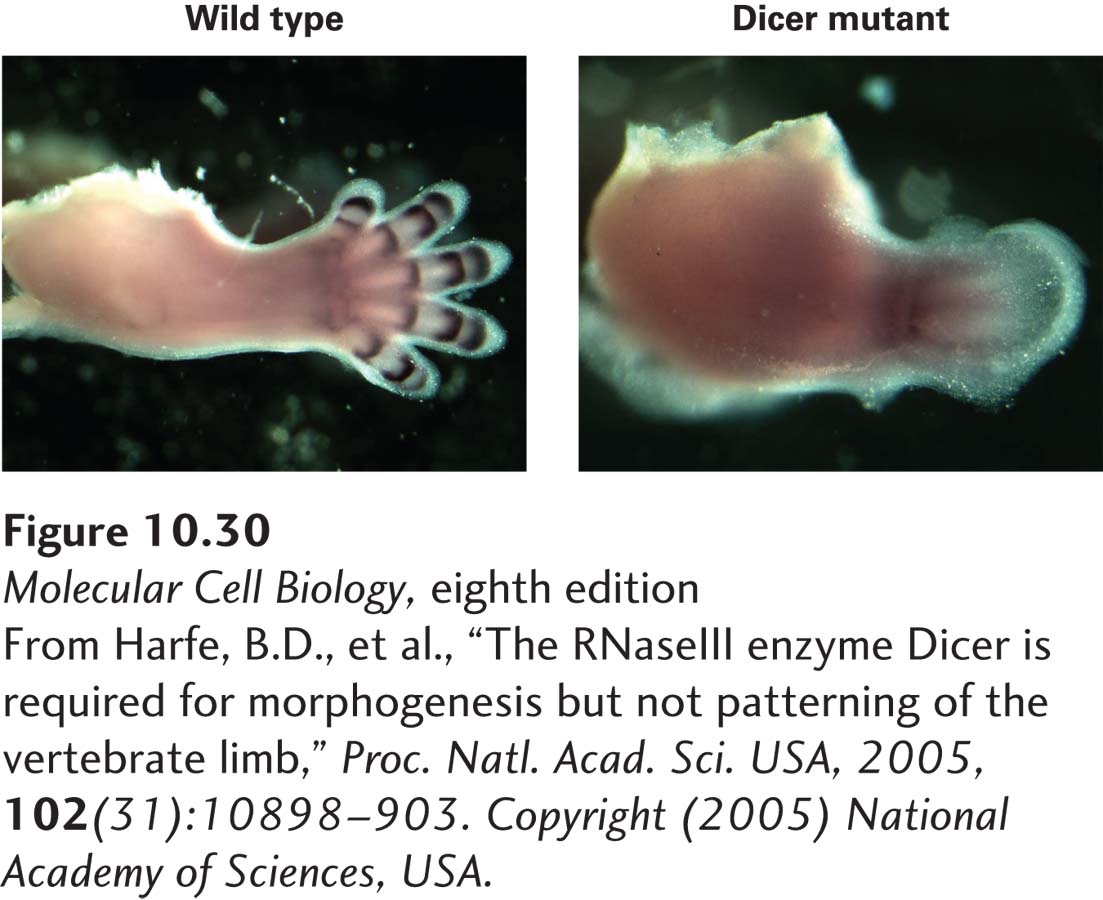
EXPERIMENTAL FIGURE 10-30 THE FUNCTION OF MIRNAS IN LIMB DEVELOPMENT. Micrographs comparing normal (left) and Dicer-knockout (right) limbs of 13-day mouse embryos immunostained for the Gd5 protein, a marker of joint formation. Dicer is knocked out in the limbs of developing mouse embryos by conditional expression of Cre to induce deletion of the dicer gene in only those cells (see Figure 6-40).
[From Harfe, B.D., et al., “The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb,” Proc. Natl. Acad. Sci. USA, 2005, 102(31):10898–903. Copyright (2005) National Academy of Sciences, USA.]


