Small Nucleolar RNAs Assist in Processing Pre-rRNAs
Ribosomal subunit assembly, maturation, and export to the cytoplasm are best understood in the yeast S. cerevisiae. However, nearly all the proteins and RNAs involved are highly conserved in multicellular eukaryotes, in which the fundamental aspects of ribosome biosynthesis are likely to be the same. Like pre-mRNAs, nascent pre-rRNA transcripts are immediately bound by proteins, forming pre-ribosomal ribonucleoprotein particles (pre-rRNPs). For reasons not yet known, cleavage of the pre-rRNA does not begin until its transcription is nearly complete. In yeast, it takes approximately 6 minutes for a pre-rRNA to be transcribed. Once transcription is complete, the pre-rRNA is cleaved, and bases and riboses are modified, in about 10 seconds. In a rapidly growing yeast cell, about 40 pairs of ribosomal subunits are synthesized, processed, and transported to the cytoplasm every second. This extremely high rate of ribosome synthesis, despite the seemingly long period required to transcribe a pre-rRNA, is possible because pre-rRNA genes are packed with RNA polymerase I molecules all transcribing the same gene simultaneously (see Figure 10-39) and because there are 100–200 such genes on chromosome XII, the yeast nucleolar organizer.
In yeast, the primary transcript of ~6.6 kb is cut in a series of cleavage and exonucleolytic steps that ultimately yield the mature rRNAs found in ribosomes (Figure 10-41). During processing, pre-rRNA is also extensively modified, mostly by methylation of the 2′-hydroxyl group of specific riboses and conversion of specific uridine residues to pseudouridine. These post-transcriptional modifications of rRNA are probably important for protein synthesis because they are highly conserved. Virtually all of these modifications occur in the most conserved core structure of the ribosome, which is directly involved in protein synthesis.
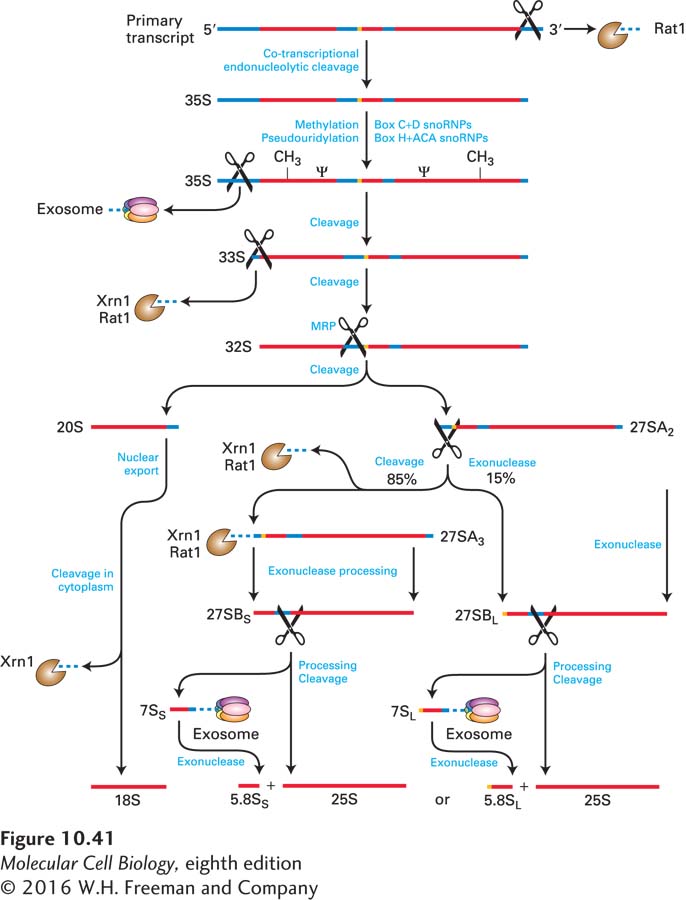
FIGURE 10-41 Pre-rRNA processing in yeast. Endoribonucleases that make internal cleavages are represented as scissors. Exoribonucleases that digest from one end, either 5′ or 3′, are shown as Pac-Men. Most 2′-O-ribose methylation (CH3) and generation of pseudouridines (Ψ) in the rRNAs occurs following the initial cleavage at the 3′ end, before the initial cleavage at the 5′ end. Proteins and snoRNPs known to participate in these steps are indicated. See J. Venema and D. Tollervey, 1999, Annu. Rev. Genet. 33:261.
The positions of the specific sites of 2′-O-methylation and pseudouridine formation are determined by approximately 150 different small nucleolus-restricted RNA species, called small nucleolar RNAs (snoRNAs), which hybridize transiently to pre-rRNA molecules. Like the snRNAs that function in pre-mRNA processing, snoRNAs associate with proteins, forming ribonucleoprotein particles called snoRNPs. One class of more than 40 snoRNPs (containing box C+D snoRNAs) positions a methyl transferase enzyme near methylation sites in the pre-rRNA. Multiple different box C+D snoRNAs direct methylation at multiple sites through a similar mechanism. They share common sequences and structural features and are bound by a common set of proteins. One or two regions of each of these snoRNAs are precisely complementary to sites on the pre-rRNA and direct the methyl transferase to specific riboses in the sequences with which they hybridize (Figure 10-42a). A second major class of snoRNPs (containing box H+ACA snoRNAs) positions the enzyme that converts uridine to pseudouridine (Figure 10-42b). This conversion involves rotation of the pyrimidine ring (Figure 10-42c). Bases on either side of the uridine to be modified in the pre-rRNA pair with bases in the bulge of a stem in the H+ACA snoRNA, leaving the uridine bulged out of the helical double-stranded region, like the branch-point A in pre-mRNA spliceosomal splicing (see Figure 10-10). Other modifications of pre-rRNA nucleotides, such as adenine dimethylation, are carried out by specific proteins without the assistance of guiding snoRNAs.
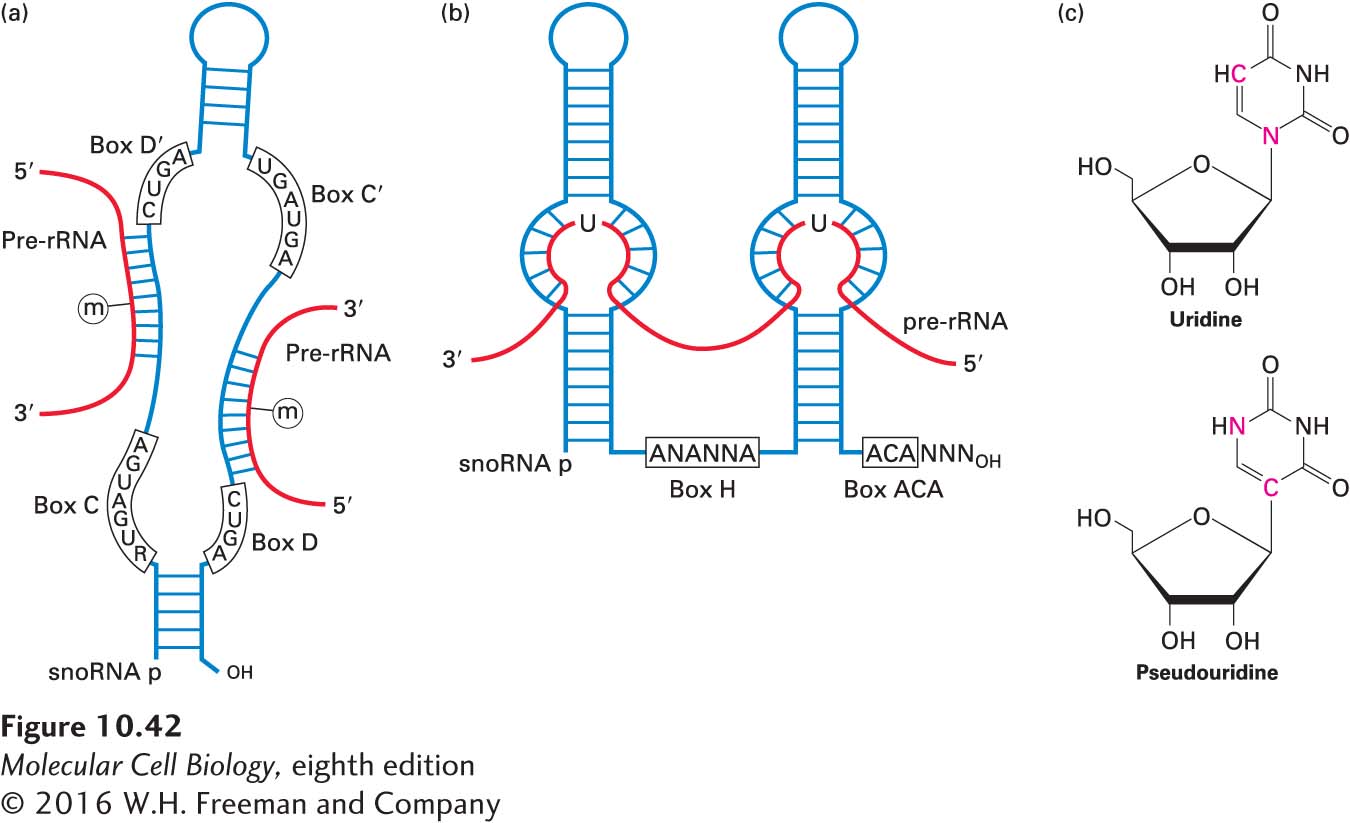
FIGURE 10-42 snoRNP-directed modification of pre-rRNA. (a) A class of snoRNAs called box C+D snoRNAs is involved in ribose 2′-O-methylation. Sequences in the snoRNA illustrated here hybridize to two different regions in the pre-rRNA, directing methylation at the indicated sites. (b) Box H+ACA snoRNAs fold into two stem-loops with internal single-stranded bulges in the stems. Pre-rRNA hybridizes to the single-stranded bulges, demarcating a site of pseudouridylation. (c) Conversion from uridine to pseudouridine involves rotation of the pyrimidine ring. See T. Kiss, 2001, EMBO J. 20:3617.
The U3 snoRNA is assembled into a large snoRNP containing some 72 proteins, called the small subunit (SSU) processome, which specifies cleavage at site A0, the initial cut near the 5′ end of the pre-rRNA (see Figure 10-41). The U3 snoRNA base-pairs with an upstream region of the pre-rRNA to specify the location of the cleavage. The processome is thought to form the “5′ knob” visible in electron micrographs of pre-rRNPs (see Figure10-39). Base pairing of other snoRNPs specifies additional cleavage reactions that remove transcribed spacer regions. The first cleavage to initiate processing of the yeast 5.8S and 25S rRNAs of the large subunit is performed by RNase MRP, a complex of nine proteins with an RNA. Once cleaved from pre-rRNAs, the spacer sequences are degraded by the same exosome-associated 3′→5′ nuclear exonucleases that degrade introns spliced from pre-mRNAs. Nuclear 5′→3′ exoribonucleases (Rat1 in yeast; XRN1 in humans) also remove some regions of 5′ spacer.
Some snoRNAs are expressed from their own promoters by RNA polymerase II or III. Remarkably, however, the large majority of snoRNAs are processed from spliced-out introns of genes encoding functional mRNAs for proteins involved in ribosome synthesis or translation. Some snoRNAs are processed from introns spliced from apparently nonfunctional mRNAs. The genes encoding these mRNAs seem to exist only to express snoRNAs from excised introns.
Unlike 18S, 5.8S, and 28S rRNA genes, 5S rRNA genes are transcribed by RNA polymerase III in the nucleoplasm outside the nucleolus. With only minor additional processing to remove nucleotides at the 3′ end, 5S rRNA diffuses to the nucleolus, where it assembles with the pre-rRNA and remains associated with the region that is cleaved into the precursor of the large ribosomal subunit.
Most of the ribosomal proteins of the small (40S) ribosomal subunit associate with the nascent pre-rRNA during transcription (Figure 10-43). Cleavage of the full-length pre-rRNA in the 90S RNP precursor of that subunit releases a pre-40S particle that requires only a few more remodeling steps before it is transported to the cytoplasm. Once the pre-40S particle leaves the nucleolus, it traverses the nucleoplasm quickly and is exported through nuclear pore complexes (NPCs), as discussed below. The final steps in the maturation of the small ribosomal subunit occur in the cytoplasm: exonucleolytic processing of the 20S rRNA into mature small subunit 18S rRNA by the cytoplasmic 5′→3′ exoribonuclease XRN1, and the dimethylation of two adjacent adenines near the 3′ end of 18S rRNA by the cytoplasmic enzyme Dim1.
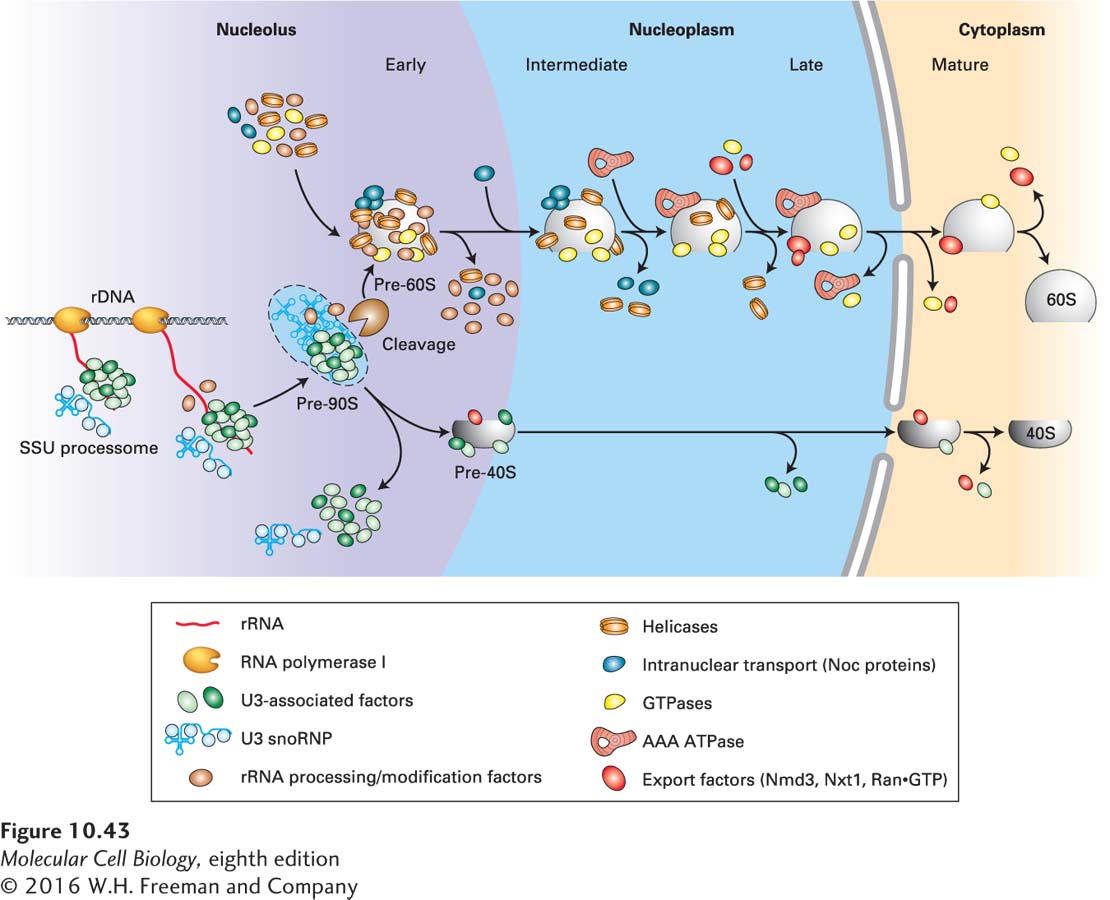
FIGURE 10-43 Ribosomal subunit assembly. Ribosomal proteins and RNAs in the maturing small and large ribosomal subunits are depicted in blue, with a shape similar to the icons for the mature subunits in the cytoplasm. Other factors that associate transiently with the maturing subunits are depicted in different colors, as shown in the key. See H. Tschochner and E. Hurt, 2003, Trends Cell Biol.13:255.
In contrast to the pre-40S particle, the precursor of the large subunit requires considerable remodeling through many more transient interactions with nonribosomal proteins before it is sufficiently mature for export to the cytoplasm. Consequently, it takes a considerably longer time for the maturing 60S subunit to exit the nucleus (30 minutes, compared with 5 minutes for export of the 40S subunit, in cultured human cells). Multiple presumptive RNA helicases and small G proteins are associated with the maturing pre-60S subunits. Some RNA helicases are necessary to dislodge the snoRNPs, which base-pair perfectly with pre-rRNA over up to 30 base pairs. Other RNA helicases may function in the disruption of protein-RNA interactions. The requirement for so many GTPases suggests that there are many quality-control checkpoints in the assembly and remodeling of the large subunit RNP, in which one step must be completed before a GTPase is activated to allow the next step to proceed. Members of the AAA ATPase family are also bound transiently. This class of proteins is often involved in large molecular movements and may be required to fold the large, complex rRNA into the proper conformation. Some steps in 60S subunit maturation occur in the nucleoplasm, during passage from the nucleolus to nuclear pore complexes (see Figure 10-43). Much remains to be learned about the complex, fascinating, and essential remodeling processes that occur during formation of the ribosomal subunits.
The large ribosomal subunit is one of the largest structures to pass through nuclear pore complexes. Maturation of the large subunit in the nucleoplasm leads to the generation of binding sites for a nuclear export adapter called Nmd3. Nmd3 is bound by the nuclear transporter exportin 1 (also called Crm1). This binding is another quality-control step because only correctly assembled subunits can bind Nmd3 and be exported. The small subunit of the mRNP exporter (Nxt1) also becomes associated with the nearly mature large ribosomal subunit. These nuclear transporters permit diffusion of the large subunit through the central channel of the NPC, which is filled with a cloud of unstructured protein domains that extend from the structured parts of the proteins that line the wall of the channel (see Chapter 13). Several additional subunits that form the walls of the NPC central channel are also required for ribosomal subunit export and may have additional functions specific for this task. The dimensions of ribosomal subunits (~25–30 nm in diameter) and the central channel of the NPC are comparable, so passage may not require distortion of either the ribosomal subunit or the channel. Final maturation of the large subunit in the cytoplasm includes removal of these export factors. Like the export of most macromolecules from the nucleus, including tRNAs and pre-miRNAs (but not most mRNPs), ribosomal subunit export requires the function of a small G protein called Ran, as discussed in Chapter 13.


