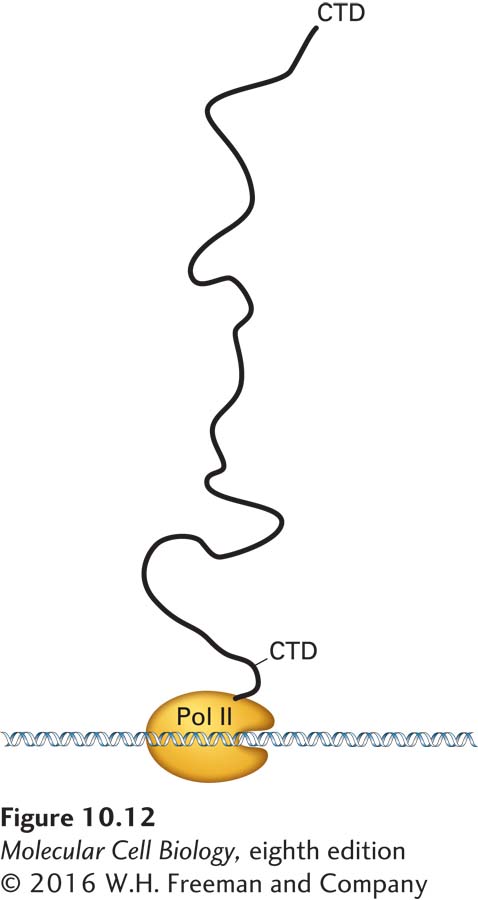
FIGURE 10-12 Schematic diagram of human RNA polymerase II with the CTD extended. The length of the human RNA polymerase II carboxy-terminal domain (CTD) and the linker region that connects it to the polymerase is shown relative to the globular domain of the polymerase. In its extended form, the CTD can associate with multiple RNA-processing factors simultaneously. See P. Cramer, D. A. Bushnell, and R. D. Kornberg, 2001, Science 292:1863.
How is RNA processing efficiently coupled with the transcription of a pre-mRNA? The key lies in the long carboxy-terminal domain (CTD) of RNA polymerase II, which, as discussed in Chapter 9, is composed of multiple repeats of a seven-residue (heptapeptide) sequence. When fully extended, the CTD domain in the human RNA polymerase II is about 130 nm long (Figure 10-12). The remarkable length of the CTD apparently allows multiple proteins to associate simultaneously with a single RNA polymerase II molecule. For instance, the enzymes that add the 5′ cap to nascent transcripts associate with the serine 5–phosphorylated CTD, as mentioned above, as do splicing and polyadenylation factors. As a consequence, these processing factors are present at high local concentrations when splice sites and polyadenylation signals are transcribed by the polymerase, enhancing the rate and specificity of RNA processing. In a reciprocal fashion, the association of hnRNP proteins with the nascent RNA enhances the interaction of RNA polymerase II with elongation factors such as DSIF and cyclin T–CDK9 (see Figure 9-21), increasing the rate of transcription. As a consequence, the rate of transcription is coordinated with the rate of nascent RNA association with hnRNPs and RNA-processing factors. This mechanism may ensure that a pre-mRNA is not synthesized unless the machinery for processing it is properly positioned.

