The Low Km of the GLUT1 Uniporter Enables It to Transport Glucose into Most Mammalian Cells
Like other uniporters, GLUT1 alternates between two conformational states: in one, a glucose-binding site faces the outside of the cell; in the other, a glucose-binding site faces the cytosol. The latter conformation has been solved at high resolution, as shown Figure 11-5a. Since the glucose concentration is usually higher in the extracellular medium (blood, in the case of erythrocytes) than in the cell, the GLUT1 uniporter generally catalyzes the net import of glucose from the extracellular medium into the cell. Figure 11-5b depicts the sequence of events during the unidirectional transport of glucose from the cell exterior inward to the cytosol through a mechanism known as the alternating access model; note the conformational changes in several of the membrane-spanning α helices during this process. GLUT1 can also catalyze the net export of glucose from the cytosol to the extracellular medium when the glucose concentration is higher inside the cell than outside.
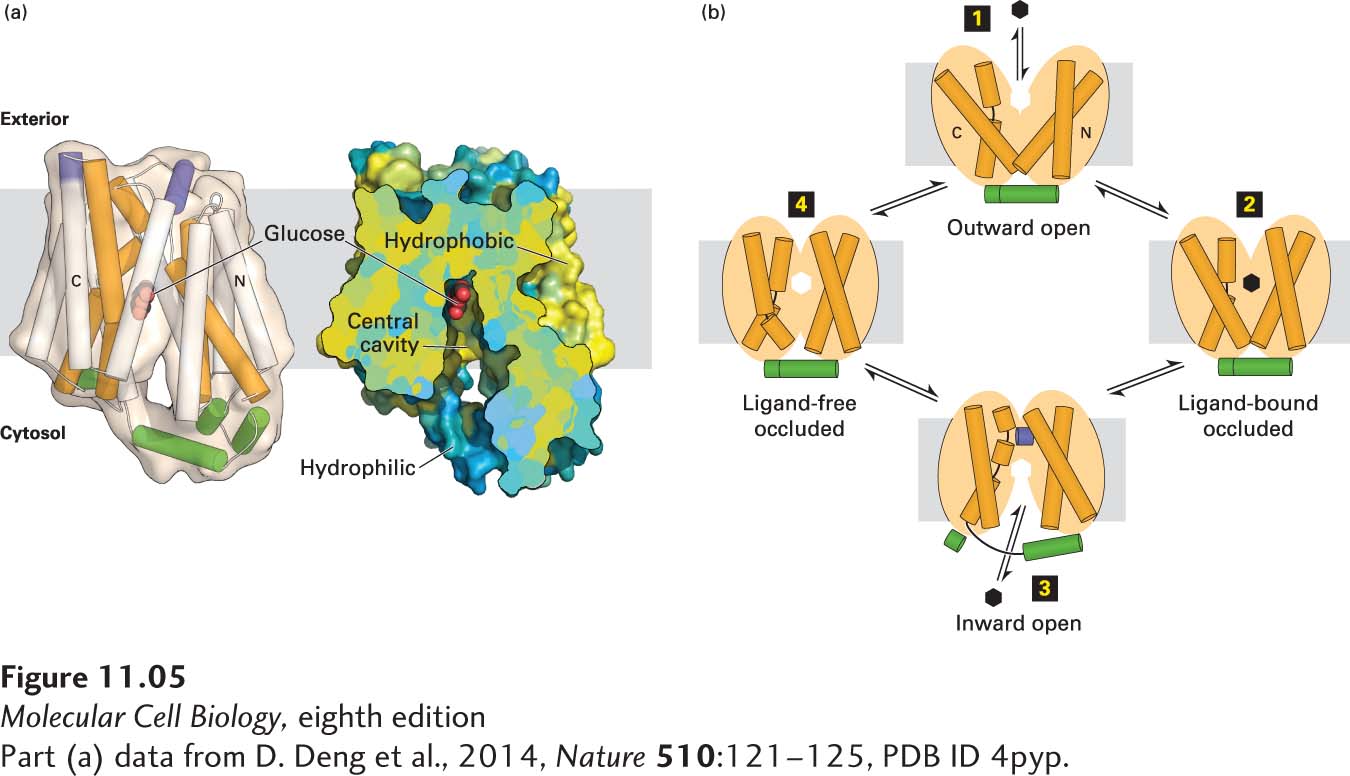
FIGURE 11-5 The human GLUT1 uniporter transports glucose across cellular membranes. (a) Structural model (side view) of the full-length human GLUT1 protein in an inward-open conformation. The transporter consists of 12 transmembrane α-helical segments, which are organized into amino-terminal and carboxy-terminal domains, each of which consists of a pair of three transmembrane α helices. The corresponding transmembrane segments in one set of the four three-helix repeats are colored orange in the model on the left. The amino-terminal and carboxy-terminal domains are connected by intracellular and extracellular α helices, which are colored green and purple, respectively. A section of a cut-open view of the surface electrostatic potential highlights the central cavity that transports glucose (red) across the membrane. The colors represent the hydrophobicity of the amino acids, with hydrophobic in yellow and hydrophilic in blue. (b) A working model for GLUT1. In this alternating access model, the outward-open conformation of GLUT1 binds glucose (step 1) and moves to a ligand-bound occluded conformation (step 2) before changing to its inward-open conformation (step 3) when it delivers glucose to the cytoplasm, then moves through a ligand-free occluded conformation (step 4) before beginning another round of glucose transport from outside to inside the cell. If the concentration of glucose is higher inside the cell than outside, the cycle will work in reverse (step 4 → step 1), resulting in net movement of glucose out of the cell. The actual conformational changes are probably smaller than those depicted here.
[Part (a) data from D. Deng et al., 2014, Nature 510:121–125, PDB ID 4pyp.]
The kinetics of the unidirectional transport of glucose from the outside of a cell inward via GLUT1 can be described by the same type of equation used to describe a simple enzyme-catalyzed chemical reaction. For simplicity, let’s assume that the substrate (glucose), S, is present initially only on the outside of the cell; this can be achieved by first incubating cells in a medium lacking glucose so that their internal stores are depleted. In this case, we can write
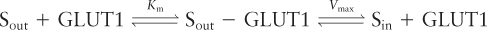
where Sout − GLUT1 represents GLUT1 in the outward-facing conformation with a bound glucose. This equation is similar to the one describing the path of a simple enzyme-catalyzed reaction in which the protein binds a single substrate and then transforms it into a different molecule. Here, however, no chemical modification of the GLUT1-bound glucose molecule occurs; rather, it is moved across a cellular membrane. Nonetheless, the kinetics of this transport reaction are similar to those of simple enzyme-catalyzed reactions, and we can use the same derivation as that of the Michaelis-Menten equation in Chapter 3 to derive the following expression for v0, the initial transport rate for S into the cell catalyzed by GLUT1:
where C is the concentration of Sout (initially, the concentration of Sin = 0). Vmax, the rate of transport when all molecules of GLUT1 contain a bound S, occurs at an infinitely high Sout concentration. The lower the value of Km, the more tightly the substrate binds to the transporter. Equation 11-1 describes the curve for glucose uptake by erythrocytes shown in Figure 11-4 as well as similar curves for other uniporters.
For GLUT1 in the human erythrocyte membrane, the Km for glucose transport is 1.5 mM. Thus when the extracellular glucose concentration is 1.5 mM, roughly half the GLUT1 transporters with outward-facing binding sites will have a bound glucose, and transport will occur at 50 percent of the maximal rate. Blood glucose is normally 5 mM, so the erythrocyte glucose transporter is usually functioning at 77 percent of its maximal rate, as can be seen from Equation 11-1. The GLUT1 transporter (or the very similar GLUT3 glucose transporter) is expressed by all body cells that need to take up glucose from the blood continuously at high rates. The rate of glucose uptake by such cells remains high regardless of small changes in the concentration of blood glucose because the blood concentration remains much higher than the Km and the intracellular glucose concentration is kept low by metabolism.
In addition to glucose, the isomeric sugars D-mannose and D-galactose, which differ from D-glucose in their configuration at only one carbon atom, are transported by GLUT1 at measurable rates. However, the Km for glucose (1.5 mM) is much lower than it is for D-mannose (20 mM) or D-galactose (30 mM). Thus GLUT1 is quite specific, having a much higher affinity (indicated by a lower Km) for its normal substrate D-glucose than for other substrates.
GLUT1 accounts for 2 percent of the protein in the plasma membrane of erythrocytes. After glucose is transported into the erythrocyte, it is rapidly phosphorylated, forming glucose-6-phosphate, which cannot leave the cell. Because this reaction, the first step in the metabolism of glucose (see Figure 12-3), is rapid and occurs at a constant rate, the intracellular concentration of glucose is kept low even when glucose is imported from the extracellular environment. Consequently, the concentration gradient of glucose (outside greater than inside the cell) is kept sufficiently high to support continuous, rapid import of additional glucose molecules and provide sufficient glucose for cellular metabolism.

