Electrons Flow “Downhill” Through a Series of Electron Carriers
Let’s examine more closely the energetically favored movement of electrons from NADH and FADH2 to the final electron acceptor, O2. For simplicity, we will focus our discussion on NADH. In respiring mitochondria, each NADH molecule releases two electrons to the electron-transport chain; these electrons ultimately reduce one oxygen atom (half of an O2 molecule), forming one molecule of water:
As electrons move from NADH to O2, their electric potential declines by 1.14 V, which corresponds to 26.2 kcal/mol of electrons transferred, or about 53 kcal/mol for a pair of electrons. As noted earlier, much of this energy is conserved in the proton-motive force generated across the inner mitochondrial membrane.
Four large multiprotein complexes (complexes I–IV) compose the electron-transport chain in the inner mitochondrial membrane that is responsible for the generation of the proton-motive force (see Figure 12-14, stage III). Each complex contains several prosthetic groups that participate in the process of moving electrons from donor molecules to acceptor molecules in coupled oxidation-reduction reactions (see Chapter 2). These small nonpeptide organic molecules or metal ions are tightly and specifically associated with the multiprotein complexes (Table 12-4).
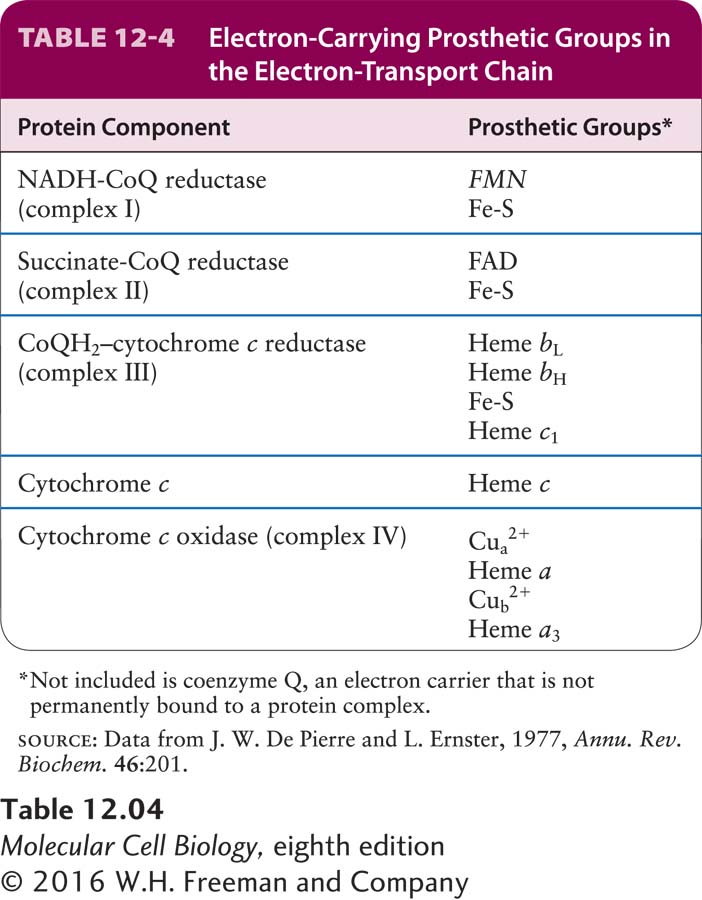
Heme and the Cytochromes Several types of heme, an iron-containing prosthetic group similar to that found in hemoglobin and myoglobin (Figure 12-20a), are tightly bound (covalently or noncovalently) to a set of mitochondrial proteins called cytochromes. Each cytochrome is designated by a letter, such as a, b, c, or c1. Electron flow through the cytochromes occurs by oxidation and reduction of the Fe atom in the center of the heme molecule:
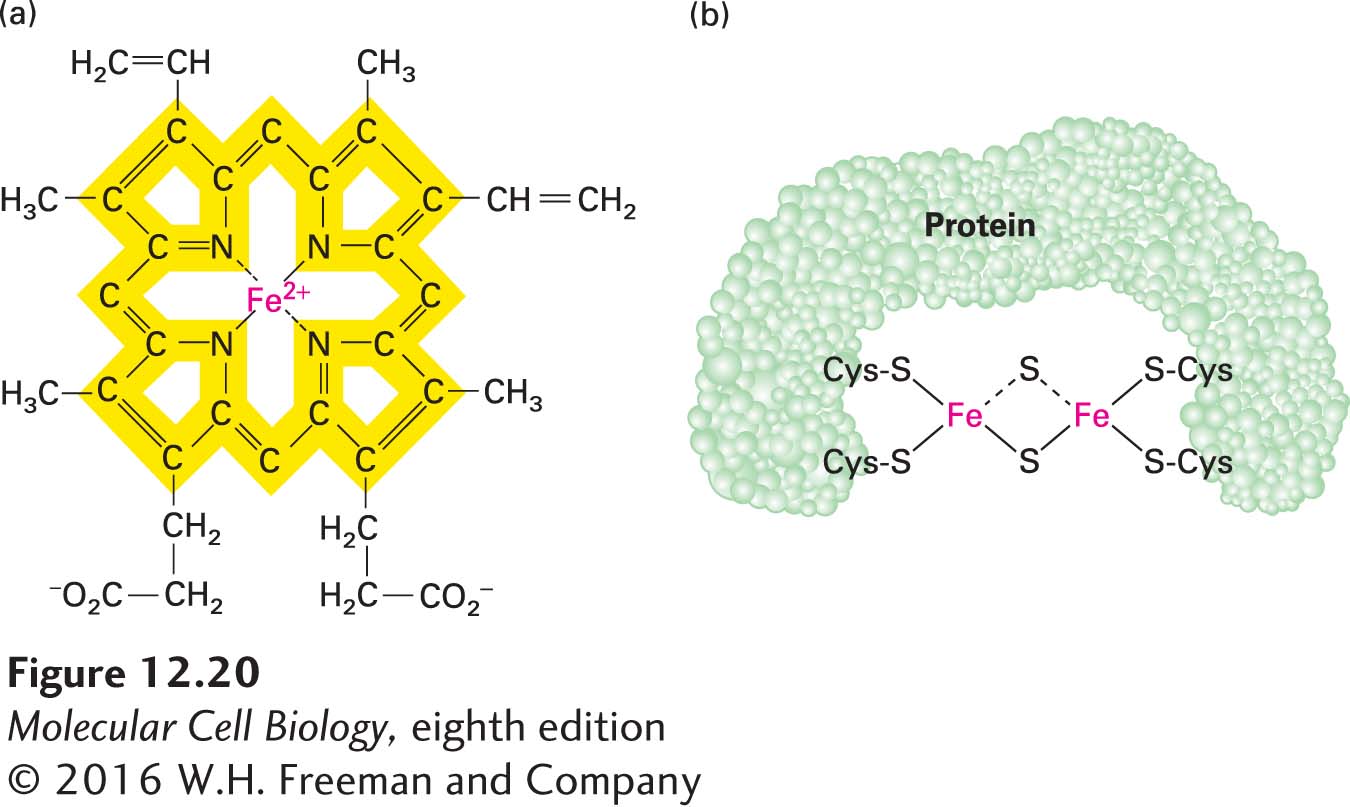
FIGURE 12-20 Heme and iron-sulfur prosthetic groups in the electron-transport chain. (a) Heme portion of cytochromes bL and bH, which are components of CoQH2–cytochrome c reductase (complex III). The same porphyrin ring (yellow) is present in all hemes. The chemical substituents attached to the porphyrin ring differ in the other cytochromes in the electron-transport chain. All hemes accept and release one electron at a time. (b) Dimeric iron-sulfur cluster (Fe-S). Each Fe atom is bonded to four S atoms: two are inorganic sulfur, and two are in cysteine side chains of the associated protein. All Fe-S clusters accept and release one electron at a time.
Because the heme ring in cytochromes consists of alternating double- and single-bonded atoms, a large number of resonance hybrid forms exist. These forms allow the extra electron delivered to the cytochrome to be spread throughout the heme carbon and nitrogen atoms as well as the Fe ion.
The various cytochromes each have slightly different heme groups and surrounding atoms (called axial ligands), which generate different environments for the Fe ion. Therefore, each cytochrome has a different reduction potential, or tendency to accept an electron—an important property that dictates the unidirectional, energetically “downhill” electron flow along the chain. Just as water spontaneously flows downhill from a higher to a lower potential energy state—but not uphill—electrons flow in only one direction from one heme (or other prosthetic group) to another due to their differing reduction potentials. (For more on the concept of reduction potential, E, see Chapter 2.) All the cytochromes except cytochrome c are components of integral membrane multiprotein complexes in the inner mitochondrial membrane.
Iron-Sulfur Clusters Iron-sulfur clusters are nonheme, iron-containing prosthetic groups consisting of Fe atoms bonded both to inorganic sulfur (S) atoms and to S atoms on cysteine residues in a protein (Figure 12-20b). Some Fe atoms in the cluster bear a +2 charge; others have a +3 charge. However, the net charge of each Fe atom is actually between +2 and +3, because electrons in their outermost orbitals, together with the extra electron delivered via the transport chain, are dispersed among the Fe atoms and move rapidly from one atom to another. Iron-sulfur clusters accept and release electrons one at a time.
Coenzyme Q Coenzyme Q (CoQ), also called ubiquinone, is the only small-molecule electron carrier in the electron-transport chain that is not an essentially irreversibly protein-bound prosthetic group (Figure 12-21). It is a carrier of both protons and electrons. The oxidized quinone form of CoQ can accept a single electron to form a semiquinone, a charged free radical denoted by CoQ•−. Addition of a second electron and two protons (thus a total of two hydrogen atoms) to CoQ•− forms dihydroubiquinone (CoQH2), the fully reduced form. Both CoQ and CoQH2 are soluble in phospholipids and diffuse freely in the hydrophobic center of the inner mitochondrial membrane. These properties underlie ubiquinone’s role in the electron-transport chain: carrying electrons and protons between the membrane-embedded protein complexes of the chain.
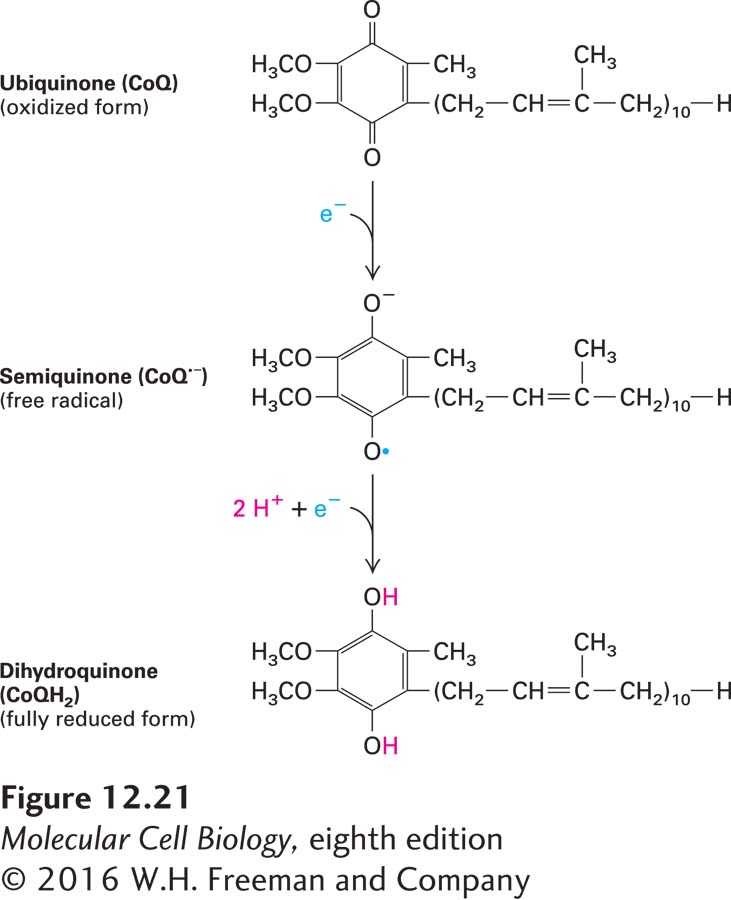
FIGURE 12-21 Oxidized and reduced forms of coenzyme Q (CoQ), which can carry two protons and two electrons. Because of its long hydrocarbon “tail” of isoprene units, CoQ, also called ubiquinone, is soluble in the hydrophobic core of phospholipid bilayers and is very mobile. Reduction of CoQ to the fully reduced form, QH2 (dihydroquinone), occurs in two steps with a half-reduced free-radical intermediate, called semiquinone.
Next we consider in detail the multiprotein complexes that use these prosthetic groups and the paths taken by electrons and protons as they pass through these complexes.


