During Glycolysis (Stage I), Cytosolic Enzymes Convert Glucose to Pyruvate
Glycolysis, the first stage of glucose oxidation, occurs in the cytosol in both eukaryotes and prokaryotes; it does not require molecular oxygen (O2) and is thus an anaerobic process. Glycolysis is an example of catabolism, the biological breakdown of complex substances into simpler ones. A set of 10 water-soluble cytosolic enzymes catalyze the reactions constituting the glycolytic pathway (glyco, “sweet”; lysis, “split”), in which one molecule of glucose is converted to two molecules of pyruvate (Figure 12-3). All the reaction intermediates produced by these enzymes are water-soluble, phosphorylated compounds called metabolic intermediates. In addition to chemically converting one glucose molecule into two pyruvates, the glycolytic pathway generates four ATP molecules by phosphorylation of four ADPs (steps 7 and 10). ATP is formed directly through the enzyme-catalyzed joining of ADP with a Pi that is derived from phosphorylated metabolic intermediates; this process is called substrate-level phosphorylation (to distinguish it from the oxidative phosphorylation that generates ATP in stages III and IV). Substrate-level phosphorylation in glycolysis, which does not involve the use of a proton-motive force, requires the prior addition (in steps 1 and 3) of two phosphates from two ATPs. These additions can be thought of as “pump priming” reactions, which introduce a little energy up front in order to effectively recover more energy downstream. Thus glycolysis yields the net production of only two ATP molecules per glucose molecule.
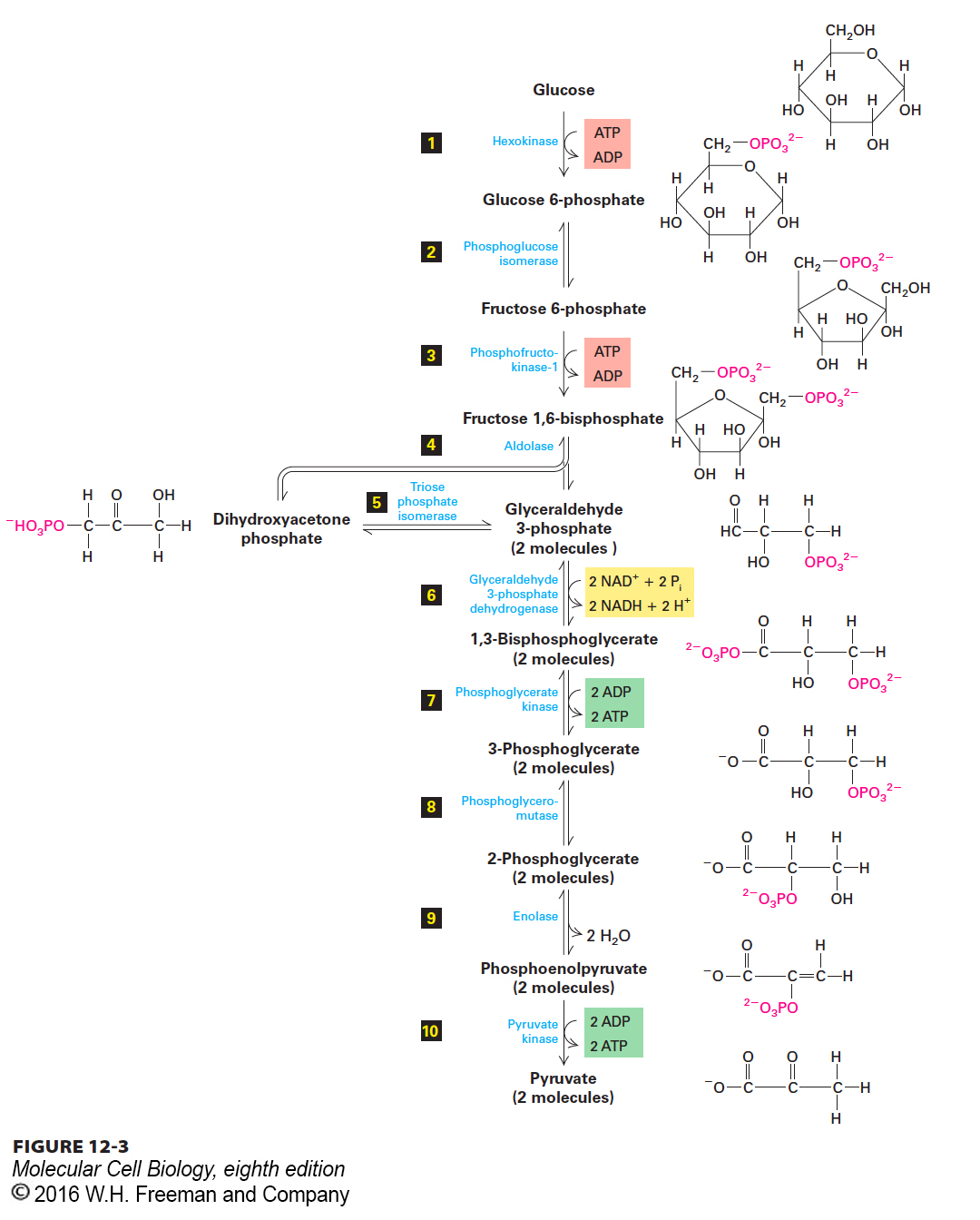
FIGURE 12-3 The glycolytic pathway. A series of ten reactions degrades glucose to pyruvate. Two reactions consume ATP, forming ADP and phosphorylated sugars (red), two generate ATP from ADP by substrate-level phosphorylation (green), and one yields NADH by reduction of NAD+ (yellow). Note that all the intermediates between glucose and pyruvate are phosphorylated compounds. Steps 1, 3 and 10, with single arrows, are essentially irreversible (have large negative ΔG values) under ordinary conditions in cells.
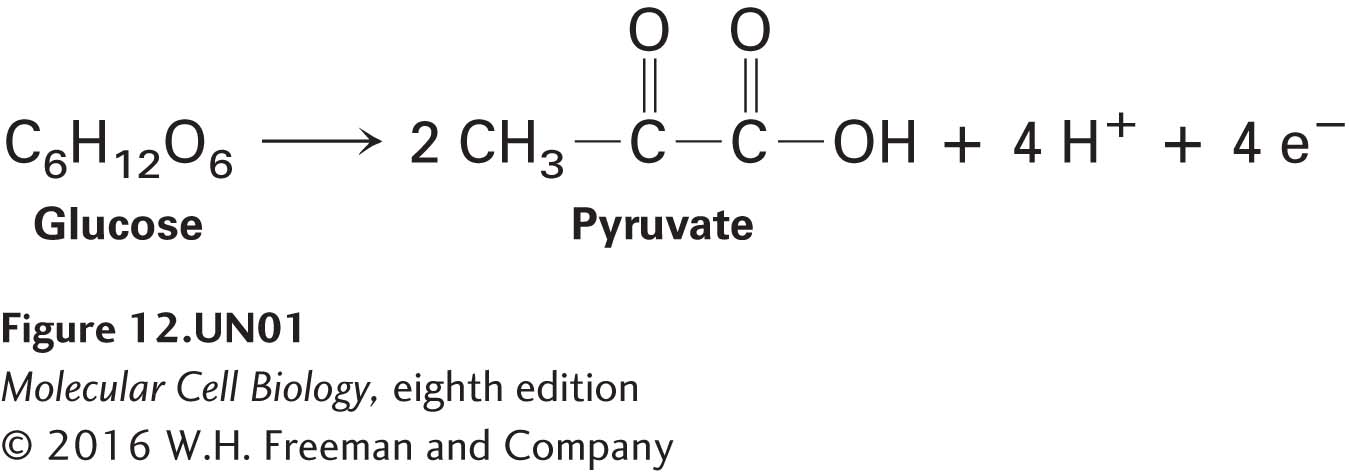
The balanced chemical equation for the conversion of glucose to pyruvate shows that four hydrogen atoms (four protons and four electrons) are also released:
(For convenience, we show pyruvate here in its un-ionized form, pyruvic acid, although at physiological pH it would be largely dissociated.) All four electrons and two of the four protons are transferred (see Figure 12-3, step 6 ) to two molecules of the oxidized form of nicotinamide adenine dinucleotide (NAD+) to produce the reduced form of the coenzyme, NADH (see Figure 2-33a):
2H+ + 4 e− + 2 NAD+ →2 NADH
Later we will see that the energy carried by the electrons in NADH and the analogous electron carrier FADH2, the reduced form of the coenzyme flavin adenine dinucleotide (FAD) (see Figure 2-33b), can be used to make additional ATPs via the electron-transport chain. The overall chemical equation for this first stage of glucose metabolism is
C6H12O6 + 2 NAD+ + 2 ADP3− + 2 Pi2− → 2 C3H4O3 + 2 NADH + 2 ATP4−
After glycolysis, only a fraction of the energy available in glucose has been extracted and converted to ATP and NADH. The rest remains trapped in the covalent bonds of the two pyruvate molecules. The ability to efficiently convert the energy remaining in pyruvate to ATP depends on the presence of molecular oxygen. As we will see, energy conversion is substantially more efficient under aerobic conditions than under anaerobic conditions.