About 1–2 percent of the oxygen metabolized by aerobic organisms, rather than being converted to water, is partially reduced to the superoxide anion radical (O2•−, where the “dot” represents an unpaired electron). Radicals are atoms that have one or more unpaired electrons in an outer (valence) shell, or molecules that contain such an atom. Many, though not all, radicals are generally highly chemically reactive, altering the structures and properties of those molecules with which they react. The products of such reactions are often themselves radicals and can thus propagate a chain reaction that alters many additional molecules. Superoxide and other highly reactive oxygen-containing molecules, both radicals (e.g.,O2•−) and non-radicals (e.g., hydrogen peroxide, H2O2), are called reactive oxygen species (ROS). ROS are of great interest because they can react with, and thus damage, many key biological molecules, including lipids (particularly unsaturated fatty acids and their derivatives), proteins, and DNA, and thus severely interfere with their normal functions. At moderate to high levels, ROS contribute to what is often called cellular oxidative stress and can be highly toxic. Indeed, ROS are purposefully generated by body-defense cells (e.g., macrophages, neutrophils) to kill pathogens. In humans, excessive or inappropriate generation of ROS has been implicated in many diverse diseases, including heart failure, neurodegenerative diseases, alcohol-induced liver disease, diabetes, and aging.
Although there are several mechanisms for generating ROS in cells, their major source in eukaryotic cells is electron transport in the mitochondria (or in chloroplasts, as described below). Electrons passing through the mitochondrial electron-transport chain can have sufficient energy to reduce molecular oxygen (O2) to form superoxide anions (Figure 12-27, top). This can occur, however, only when molecular oxygen comes in close contact with the reduced electron carriers (iron, FMN, CoQH2) in the chain. Usually such contact is prevented by sequestration of the carriers within the proteins involved. However, there are some sites (particularly in complex I and CoQ•−, see Figure 12-21) and some conditions (e.g., high NADH/NAD+ ratio in the matrix, high proton-motive force when ATP is not generated) when electrons can more readily “leak” out of the chain and reduce O2 to O2•−.
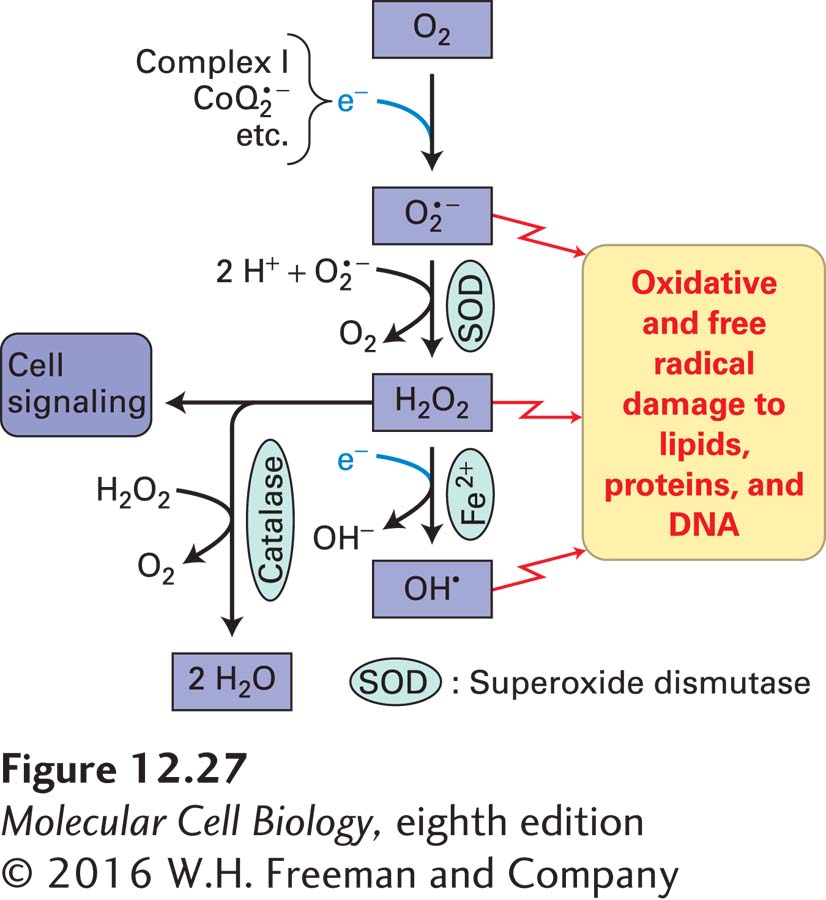
FIGURE 12-27 Generation and inactivation of toxic reactive oxygen species. Electrons from the electron-transport chains of mitochondria and chloroplasts, as well as some generated through other enzymatic reactions, reduce molecular oxygen (O2), forming the highly reactive radical anion superoxide (O2•−). Superoxide is rapidly converted by superoxide dismutase (SOD) to hydrogen peroxide (H2O2), which in turn can be converted by metal ions such as Fe2+ to hydroxyl radicals (OH•) or inactivated to H2O by enzymes such as catalase. Because of their high chemical reactivity, O2•−, H2O2, OH•, and similar molecules are called reactive oxygen species (ROS). They cause oxidative and free-radical damage to many biomolecules, including lipids, proteins, and DNA. This damage leads to cellular oxidative stress that can cause disease and, if sufficiently severe, can kill cells. In addition, ROS can function as intra- and intercellular signaling molecules.
The superoxide anion is an especially unstable and reactive ROS. Mitochondria have evolved several defense mechanisms that help protect against O2•− toxicity, including the use of enzymes that inactivate superoxide, first by converting it to H2O2 (Mn-containing superoxide dismutase, called SOD) and then to H2O (catalase) (see Figure 12-27). Because O2•− is so highly reactive and toxic, SOD and catalase are some of the fastest enzymes known so that they prevent the buildup of these ROS. SOD is found within mitochondria and other cellular compartments. Hydrogen peroxide itself is a ROS that can diffuse readily across membranes and react with molecules throughout the cell. It can also be converted by certain metals, such as Fe2+, into the even more dangerous hydroxyl radical (OH•). Thus cells depend on the inactivation of H2O2 by catalase and other enzymes, such as peroxiredoxin and glutathione peroxidase, which also detoxify the lipid hydroperoxide products formed when ROS react with unsaturated fatty acyl groups. Small-molecule antioxidant radical scavengers, such as vitamin E and α-lipoic acid, also protect against oxidative stress. Although in many cells catalase is located only in peroxisomes, in heart muscle cells it is found in mitochondria. This is not surprising because the heart is the most oxygen-consuming organ per gram in mammals.
As the rate of ROS production by mitochondria and chloroplasts reflects the metabolic state of these organelles (e.g., strength of proton-motive force, NADH/NAD+ ratio), cells have developed ROS-sensing systems, such as ROS/redox-sensitive transcription factors, to monitor the metabolic state of these organelles and respond accordingly—for example, by changing the rate of transcription of nuclear genes that encode organelle-specific proteins. There are also reports that H2O2 can function as a physiologically relevant intra- and intercellular signaling molecule. ROS have been reported to participate in cell processes as diverse as adaptation to low oxygen levels (hypoxia) and stress, growth factor and nutrient regulation of cell proliferation, cell differentiation, regulated cell death, and autophagy.
