Experiments Using Purified Electron-Transport Chain Complexes Established the Stoichiometry of Proton Pumping
The multiprotein complexes of the electron-transport chain that are responsible for proton pumping have been identified by selectively extracting mitochondrial membranes with detergents, isolating each of the complexes in nearly pure form, and then preparing artificial phospholipid vesicles (liposomes) containing each complex. When an appropriate electron donor and electron acceptor are added to such liposomes, a change in the pH of the medium will occur if the embedded complex transports protons (Figure 12-28). Studies of this type indicate that NADH-CoQ reductase (complex I) translocates four protons per pair of electrons transported, whereas cytochrome c oxidase (complex IV) translocates two protons per pair of electrons transported.
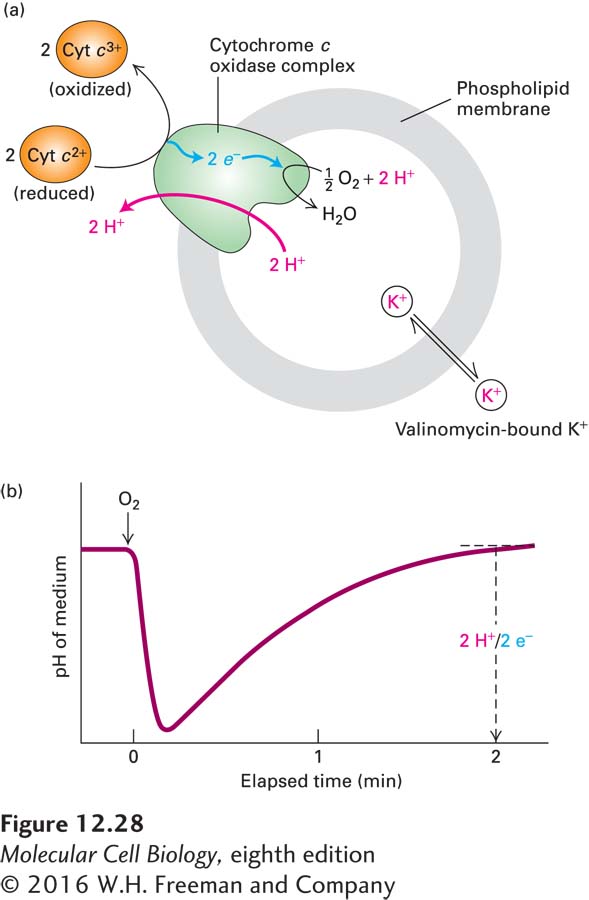
EXPERIMENTAL FIGURE 12-28 Electron transfer from reduced cytochrome c to O2 via cytochrome c oxidase (complex IV) is coupled to proton transport. The cytochrome c oxidase complex is incorporated into liposomes with the binding site for cytochrome c positioned on the outer surface. (a) When O2 and reduced cytochrome c are added, electrons are transferred to O2 to form H2O, and protons are transported from the inside to the medium outside the vesicles. A drug called valinomycin is added to the medium to dissipate the voltage gradient generated by the translocation of H+, which would otherwise reduce the number of protons moved across the membrane. (b) Monitoring of the medium’s pH reveals a sharp drop in pH following addition of O2. As the reduced cytochrome c becomes fully oxidized, protons leak back into the vesicles, and the pH of the medium returns to its initial value. Measurements show that two protons are transported per O atom reduced. Two electrons are needed to reduce one O atom, but cytochrome c transfers only one electron; thus two molecules of cytochrome c2+ are oxidized for each O reduced. See B. Reynafarje et al., 1986, J. Biol. Chem. 261:8254.
Current evidence suggests that a total of ten protons are transported from the matrix across the inner mitochondrial membrane for every electron pair that is transferred from NADH to O2 (see Figure 12-22). Because succinate-CoQ reductase (complex II) does not transport protons, and because complex I is bypassed when the electrons come from succinate-derived FADH2, only six protons are transported across the membrane for every electron pair that is transferred from this FADH2 to O2.
