The Proton-Motive Force in Mitochondria Is Due Largely to a Voltage Gradient Across the Inner Membrane
The main result of the electron-transport chain is the generation of the proton-motive force, which is the sum of a transmembrane proton concentration (pH) gradient and an electric potential, or voltage gradient, across the inner mitochondrial membrane. The relative contributions of these two components to the total proton-motive force have been shown to depend on the permeability of the membrane to ions other than H+. A significant voltage gradient can develop only if the membrane is poorly permeable to other cations and to anions. Otherwise, anions would leak across the membrane from the matrix to the intermembrane space along with the protons and prevent a voltage gradient from forming. Similarly, if cations other than H+ could leak across the membrane in a direction opposite to that of the H+ (from the intermembrane space to the matrix), that leakage would counterbalance the charge delivered to the intermembrane space by the protons, short-circuiting voltage-gradient formation. Indeed, the inner mitochondrial membrane is poorly permeable to ions other than H+. Thus proton pumping generates a voltage gradient that makes it energetically difficult for additional protons to move across the membrane because of charge repulsion. As a consequence, proton pumping by the electron-transport chain establishes a robust voltage gradient in the context of what turns out to be a rather small pH gradient.
Because mitochondria are much too small to be impaled with electrodes, the electric potential and pH gradient across the inner mitochondrial membrane cannot be directly measured. However, the electric potential can be measured indirectly by adding radioactive 42K+ ions and a trace amount of valinomycin to a suspension of respiring mitochondria and measuring the amount of radioactivity that accumulates in the matrix. Although the inner membrane is normally impermeable to K+, valinomycin is an ionophore, a small lipid-soluble molecule that selectively binds a specific ion (in this case, K+) and carries it across otherwise impermeable membranes. In the presence of valinomycin, 42K+ equilibrates across the inner membrane of isolated mitochondria in accordance with the electric potential: the more negative the matrix side of the membrane, the more 42K+ will be attracted to and accumulate in the matrix.
At equilibrium, the measured concentration of radioactive K+ ions in the matrix, [Kin], is about 500 times greater than that in the surrounding medium, [Kout]. Substitution of this value into the Nernst equation (see Chapter 11) shows that the electric potential E (in mV) across the inner membrane in respiring mitochondria is −160 mV, with the matrix (inside) negative:
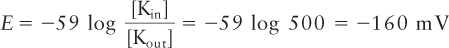
Researchers can measure the matrix (inside) pH by trapping pH-sensitive fluorescent dyes inside vesicles formed from the inner mitochondrial membrane, with the matrix side of the membrane facing inward. They can also measure the pH outside the vesicles (equivalent to the intermembrane space) and thus determine the pH gradient (ΔpH), which turns out to be about one pH unit. A difference of one pH unit represents a tenfold difference in H+ concentration, so according to the Nernst equation, a pH gradient of one unit across a membrane is equivalent to an electric potential of 59 mV at 20 °C. Thus, knowing the voltage and pH gradients, we can calculate the proton-motive force (pmf) as
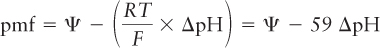
where R is the gas constant of 1.987 cal/(degree · mol), T is the temperature (in degrees Kelvin), F is the Faraday constant [23,062 cal/(V · mol)], and Ψ is the transmembrane electric potential; Ψ and pmf are measured in millivolts. The electric potential Ψ across the inner membrane is −160 mV (negative inside), and ΔpH is equivalent to about 60 mV. Thus the total proton-motive force is −220 mV, with the transmembrane electric potential responsible for about 73 percent of the total.